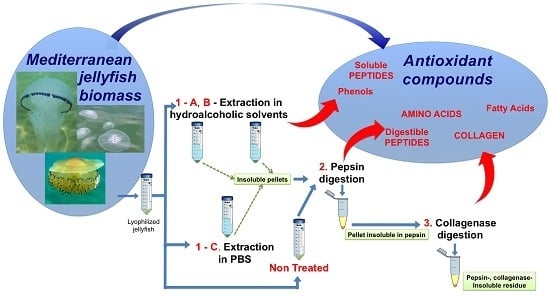
The Bright Side of Gelatinous Blooms: Nutraceutical Value and Antioxidant Properties of Three Mediterranean Jellyfish (Scyphozoa)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Jellyfish Blooms and Biomass Characterization
| Jellyfish Samples | Umbrella Diameter Range * | Fresh Weight Range * | Ratio FW/Diameter | Range of DW * | Organic Matter (OM) Mean ** |
|---|---|---|---|---|---|
| Mean (cm) | Mean (g) | (% of FW) | (% of DW) | ||
| Aurelia sp.1 | 10–23 16.2 ± 4.9 | 47–604 257 ± 237 | 6.8–26.3 13.5 ± 9.2 | 2.2–3.0 | 23.9 ± 3.3 a |
| Cotylorhiza tuberculata | 6–29 17.7 ± 6.3 | 19–1770 638 ± 475 | 3–61 24.3 ± 16.9 | 3.9–32.4 | 30.2 ± 2.4 b |
| Rhizostoma pulmo | 8–37 20.8 ± 7.2 | 42–2440 860 ± 720 | 5.3–65.9 32.6 ± 14.2 | 4.1–6.8 | 29.5 ± 6.6 b |
2.2. Jellyfish Protein
2.2.1. Amino Acid Composition
| Aurelia sp.1 | C. tuberculata | R. pulmo | ||||
|---|---|---|---|---|---|---|
| mg/100 g ± SD | % | mg/100 g ± SD | % | mg/100 g ± SD | % | |
| Alanine (Ala) | 7.1 ± 0.3 | 4.5 | 2.2 ± 0.2 | 4.3 | 3.5 ± 0.2 | 3.9 |
| Arginine (Arg) | 1.1 ± 0.0 | 0.7 | n.d. | - | 1.8 ± 0.0 | 2.0 |
| Aspartic acid + Asparagine (Asx) * | 3.2 ± 0.2 | 2.0 | 1.3 ± 0.1 | 2.5 | 2.9 ± 0.6 | 3.2 |
| Cysteine (Cys) | 4.1 ± 0.2 | 2.6 | n.d. | - | 1.2 ± 0.0 | 1.3 |
| Glutamic acid + Glutamine (Glx) ** | 13.6 ± 0.3 | 8.7 | 8.2 ± 0.6 | 16.0 | 13.7 ± 0.2 | 15.2 |
| Glycine (Gly) | 55.4 ± 1.1 | 35.2 | 3.0 ± 0.1 | 5.9 | 4.8 ± 0.5 | 5.3 |
| Histidine (His) e | n.d. | - | 4.0 ± 0.1 | 7.8 | 5.0 ± 0.4 | 5.6 |
| Isoleucine (Ile) e | 6.8 ± 0.3 | 4.3 | 2.9 ± 0.5 | 5.7 | 4.9 ± 0.7 | 5.5 |
| Leucine (Leu) e | n.d. | - | 3.8 ± 0.6 | 7.4 | 8.2 ± 0.4 | 9.1 |
| Lysine (Lys) e | 9.4 ± 0.3 | 6.0 | 3.1 ± 0.5 | 6.1 | 6.2 ± 0.4 | 6.9 |
| Methionine (Met) e | 5.9 ± 0.9 | 3.8 | 2.7 ± 0.5 | 5.3 | 4.1 ± 0.7 | 4.6 |
| Phenylalanine (Phe) e | 10.4 ± 0.3 | 6.6 | 4.1 ± 0.2 | 8.0 | 8.4 ± 0.8 | 9.3 |
| Proline (Pro) | 4.3 ± 0.3 | 2.7 | 2.6 ± 0.3 | 5.1 | 3.5 ± 0.2 | 3.9 |
| Serine (Ser) | 9.5 ± 0.4 | 6.0 | 2.8 ± 0.0 | 5.5 | 6.0 ± 0.8 | 6.7 |
| Threonine (Thr) e | 10.1 ± 0.9 | 6.4 | 3.8 ± 0.0 | 7.4 | 4.5 ± 0.1 | 5.0 |
| Tyrosine (Tyr) | 9.5 ± 0.2 | 6.0 | 3.6 ± 0.2 | 7.0 | 6.8 ± 0.6 | 7.6 |
| Tryptophan (Try) e | n.d. | - | n.d. | - | n.d. | - |
| Valine (Val) e | 6.8 ± 0.5 | 4.3 | 3.0 ± 0.3 | 5.9 | 4.4 ± 0.4 | 4.9 |
| ∑AA | 157.2 ± 6.2 | 100 | 51.1 ± 4.2 | 100 | 89.9 ± 7.0 | 100 |
| ∑EAA | 49.4 ± 3.2 | 31.4 | 27.4 ± 2.7 | 53.6 | 45.7 ± 3.9 | 50.8 |
| ∑CAA | 93.4 ± 2.3 | 59.4 | 20.2 ± 1.2 | 39.5 | 36.6 ± 2.3 | 40.7 |
| ∑AAA | 19.9 ± 0.5 | 12.7 | 11.7 ± 0.5 | 22.9 | 20.2 ± 1.8 | 22.5 |
2.2.2. Protein Content and Composition
| Aurelia sp.1 | Cotylorhiza tuberculata | Rhizostoma pulmo | Collagen (Chicken Cartilage) (%) | ||
|---|---|---|---|---|---|
| Treatments | mg of proteins/g of dry weight (% of total proteins) | ||||
| Soluble proteins | A—80% MeOH B—80% EtOH C—PBS | 3.8 ± 0.8 (6.9) 3.9 ± 0.4 (7.7) 22.3 ± 1.1 (32.2) | 2.4 ± 0.5 (8.3) 2.8 ± 0.8 (10.5) 35.4 ± 4.6 (59.1) | 2.0 ± 0.9 (3.4) 2.1± 1.0 (3.4) 37.4 ± 6.6 (38.5) | - - - |
| Pepsin digestible proteins | Treatment A Treatment B Treatment C Non Treated | 12.2 ± 2.9 (22.1) 10.4 ± 0.9 (20.6) 7.3 ± 0.2 (10.6) 14.5 ± 2.0 (25.5) | 6.4 ± 3.2 (22.2) 6.7 ± 3.2 (25.1) 4.5 ± 1.4 (7.6) 4.3 ± 0.9 (19.4) | 18.6 ± 3.0 (32.4) 20.6 ± 3.5 (33.4) 19.9 ± 4.4 (20.5) 19.6 ± 4.7 (32.5) | (0.7) |
| Collagenase hydrolysable proteins | Treatment A Treatment B Treatment C Non Treated | 38.0 ± 0.6 (69.1) 35.3 ± 0.9 (69.9) 38.8 ± 1.8 (55.8) 40.5 ± 0.5 (71.0) | 18.4 ± 0.8 (63.1) 14.6 ± 0.8 (55.0) 18.3 ± 0.7 (30.6) 15.7 ± 4.4 (70.8) | 33.6 ± 5.1 (58.6) 35.6 ± 4.3 (57.6) 35.2 ± 6.9 (36.2) 36.6 ± 7.0 (60.8) | (99.3) |
| Not-hydrolyzed proteins | Treatment A Treatment B Treatment C Non Treated | 1.1 ± 0.1 (1.9) 1.9 ± 0.1 (1.8) 1.0 ± 0.1 (1.5) 2.0 ± 0.2 (3.6) | 1.9 ± 0.3 (6.4) 2.5 ± 0.4 (9.5) 1.6 ± 0.1 (2.8) 2.2 ± 0.3 (9.8) | 3.2 ± 0.3 (5.6) 3.5 ± 0.9 (5.6) 4.7 ± 1.3 (4.9) 4.0 ± 0.9 (6.7) | - |
| Total | Treatment A Treatment B Treatment C Non Treated | 55.1 (100) 50.5 (100) 69.5 (100) 57.0 (100) | 29.1 (100) 26.6 (100) 59.9 (100) 22.2 (100) | 57.5 (100) 61.8 (100) 97.2 (100) 60.1 (100) | (100) |

2.3. Phenolic Compound Content in Jellyfish Hydroalcoholic Soluble Extracts

2.4. Antioxidant Activity


2.4.1. Antioxidant Activity in Aqueous and Hydroalcoholic Extracts
2.4.2. Antioxidant Activity of Enzymatic Hydrolyzed Peptides
2.5. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis of Hydrolyzed Peptides

2.6. Lipid Content
| Aurelia sp1 | Cotylorhiza tuberculata | Rhizostoma pulmo | |
|---|---|---|---|
| Fatty acids (FA) | % | ||
| Saturated FA (SFA) | |||
| Lauric acid C12:0 | - | - | 1.3±0.5 |
| Myristic acid C14:0 | 2.4 ± 0.6 | 2.9 ± 0.2 | 3.1 ± 0.4 |
| Palmitic acid C16:0 | 33.0 ± 1.9 | 26.1 ± 0.1 | 33.2 ± 0.5 |
| Margaric acid C17:0 | 1.4 ± 0.5 | 0.8 ± 0.1 | - |
| Stearic acid C18:0 | 32.7 ± 1.6 | 24.2 ± 0.5 | 30.6 ± 1.8 |
| Arachidic acid C20:0 | - | 0.8 ± 0.1 | - |
| Total SFA | 69.5 | 54.8 | 68.2 |
| Monounsaturated FA | |||
| Palmitoleic acid C16:1 | - | 1.2 ± 0.7 | |
| Oleic acid C18:1 (ω9) | 3.0 ± 0.7 | 12.8 ± 0.1 | 5.1 ± 1.8 |
| Vaccenic acid C18:1 (ω7) | 1.7 ± 0.2 | 1.2 ± 0.1 | 1.9 ± 0.5 |
| Total MUFA | 4.7 | 15.2 | 7.0 |
| Polyunsaturated FA (PUFA) | |||
| Linoleic acid C18:2 (ω6) * | 1.3 ± 0.2 | 8.3 ± 1.6 | 2.5 ± 0.7 |
| Eicosatetraenoic acid C20:4 (ω3) | - | 4. 1 ± 0.3 | - |
| Arachidonic acid C20:4 (ω6) | 5.5 ± 1.1 | 5.3 ± 0.5 | 8.8 ± 0.5 |
| Eicosapentaenoic acid C20:5 (ω3) | 14.6 ± 2.2 | 5.1 ± 0.5 | 8.6 ± 1.7 |
| Docosahexaenoic acid C22:6 (ω3) | 4.4 ± 1.1 | 7.2 ± 0.9 | 4.9 ± 1.1 |
| Total PUFA | 25.8 | 30.0 | 24.8 |
| Σω6 | 6.8 | 13.6 | 11.3 |
| Σω3 | 19.0 | 16.4 | 13.5 |
| ω6/ω3 | 0.36 | 0.83 | 0.8 |
| Total Lipids (g/100 g dry weight) | 4.1 ± 0.5 | 12.3 ± 0.7 | 4.0 ± 0.8 |
3. Experimental Section
3.1. Materials and Chemicals
3.2. Sample Collection and Preparation
3.3. Sequential Extraction and Hydrolysis
3.3.1. Polar Solvent Extraction
3.3.2. Enzymatic Hydrolyses

3.4. Protein Content
3.5. Amino Acidic Composition Analysis
3.6. Phenol Content
3.7. Antioxidant Activity
3.8. SDS-PAGE
3.9. Total Lipid Extraction
Fatty Acid Profiles Determination
3.10. GC-MS Analysis
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gates, K.W. Marine Products for Healthcare: Functional and Bioactive Nutraceutical Compounds from the Ocean, Vazhiyil Venugopal. J. Aquat. Food Prod. Technol. 2010, 19, 48–54. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2014, 31, 160–258. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, J.; Hu, G.; Yu, J.; Zhu, X.; Lin, Y.; Chen, S.; Yuan, J. Statistical Research on the Bioactivity of New Marine Natural Products Discovered during the 28 Years from 1985 to 2012. Mar. Drugs 2015, 13, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Jaume, D.; Duarte, C.M. General aspects concerning marine and terrestrial biodiversity. In The Exploration of Marine Biodiversity Scientific and Technological Challenges; Duarte, C.M., Ed.; Fundatiòn BBVA: Bilbao, Spain, 2006; pp. 19–32. [Google Scholar]
- Mayer, A.M.S.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine pharmacology in 2009–2011: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2013, 11, 2510–2573. [Google Scholar] [CrossRef] [PubMed]
- Blunden, G. Biologically active compounds from marine organisms. Phyther. Res. 1990, 15, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Guérard, F.; Decourcelle, N.; Sabourin, C.; Floch-Laizet, C.; le Grel, L.; le Floch, P.; Gourlay, F.; le Delezir, R.; Jaouen, P.; Bourseau, P. Recent developments of marine ingredients for food and nutraceutical applications: A review. J. Sci. Halieut. Aquat. 2010, 2, 21–27. [Google Scholar]
- Leone, A.; Lecci, R.M.; Durante, M.; Piraino, S. Extract from the zooxanthellate jellyfish Cotylorhiza tuberculata modulates gap junction intercellular communication in human cell cultures. Mar. Drugs 2013, 11, 1728–1762. [Google Scholar] [CrossRef] [PubMed]
- Mariottini, G.L.; Pane, L. Cytotoxic and cytolytic cnidarian venoms. A review on health implications and possible therapeutic applications. Toxins (Basel) 2013, 6, 108–151. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D.; Watson, R.; Alder, J. Global trends in world fisheries: Impacts on marine ecosystems and food security. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Bundy, A.; Shannon, L.J.; Rochet, M.-J.; Neira, S.; Shi, Y.-J.; Hill, L.; Aydin, K. The Good (ish), the Bad and the Ugly: A tripartite classification of ecosystem trends. ICES J. Mar. Sci. 2010, 67, 745–768. [Google Scholar] [CrossRef]
- Boero, F.; Bouillon, J.; Gravili, C.; Miglietta, M.P.; Parsons, T.; Piraino, S. Gelatinous plankton: Irregularities rule the world (sometimes). Mar. Ecol. Prog. Ser. 2008, 356, 299–310. [Google Scholar] [CrossRef]
- Graham, W.M.; Pagès, F.; Hamner, W.M. A physical context for gelatinous zooplankton aggregations: A review. Hydrobiologia 2001, 451, 199–212. [Google Scholar] [CrossRef]
- Brotz, L.; Cheung, W.W.L.; Kleisner, K.; Pakhomov, E.; Pauly, D. Increasing jellyfish populations: Trends in Large Marine Ecosystems. Hydrobiologia 2012, 690, 3–20. [Google Scholar] [CrossRef]
- Mariottini, G.L.; Pane, L. Mediterranean jellyfish venoms: A review on scyphomedusae. Mar. Drugs 2010, 8, 1122–1152. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Liu, D.; Keesing, J.K. Jellyfish blooms in China: Dominant species, causes and consequences. Mar. Pollut. Bull. 2010, 60, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.H.; Gelcich, S.; Uye, S.I. Living with jellyfish: Management and adaptation strategies. In Jellyfish Blooms; Pitt, K.A., Lucas, C.H., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 129–150. [Google Scholar]
- Purcell, J.E.; Uye, S.I.; Lo, W.T. Anthropogenic causes of jellyfish blooms and their direct consequences for humans: A review. Mar. Ecol. Prog. Ser. 2007, 350, 153–174. [Google Scholar] [CrossRef]
- Lynam, C.P.; Gibbons, M.J.; Axelsen, B.E.; Sparks, C.A.J.; Coetzee, J.; Heywood, B.G.; Brierley, A.S. Jellyfish overtake fish in a heavily fished ecosystem. Curr. Biol. 2006, 16, 1976. [Google Scholar] [CrossRef]
- Purcell, J.E.; Milisenda, G.; Rizzo, A.; Carrion, S.A.; Zampardi, S.; Airoldi, S.; Zagami, G.; Guglielmo, L.; Boero, F.; Doyle, T.K.; Piraino, S. Digestion and predation rates of zooplankton by the pleustonic hydrozoan Velella velella and widespread blooms in 2013 and 2014. Plankt. Res. 2015, in press. [Google Scholar] [CrossRef]
- Matsushita, Y.; Honda, N.; Kawamura, S. Design and tow trial of JET (Jellyfish Excluder for Towed fishing gear). Nippon Suisan Gakk. 2005, 71, 965–967. [Google Scholar] [CrossRef]
- MED-JELLYRISK, Enhancing management approach and mitigation measures against jellyfish proliferations impacts. Project funded by the ENPI CBC MED (European Neighbourhood and Partnership Instrument Cross-Border Cooperation in the Mediterranean). Available online: http://meteomeduse.focus.it/ (accessed on 23 December 2013).
- Boero, F. Review of jellyfish blooms in the Mediterranean and Black Sea. In General Fisheries Commission for the Mediterranean. Studies and Reviews; No. 92; Food and Agriculture Organization of the United Nations (FAO), Ed.; FAO: Rome, Italy, 2013; p. 53. [Google Scholar]
- Doyle, T.K.; Hays, G.C.; Harrod, C.; Houghton, J.D.R. Ecological and societal benefits of jellyfish. In Jellyfish Blooms; Pitt, K.A., Lucas, C.H., Eds.; Springer Science + Business Media: Dordrecht, The Netherlands, 2014; pp. 105–127. [Google Scholar]
- Boero, F.; Bouillon, J.; Piraino, S.; Schmid, V. Asexual reproduction in the Hydrozoa (Cnidaria). In Reproductive Biology of Invertebrates XI: Progress in Asexual Reproduction; Hughes, R.N., Ed.; Oxford & IBH Publishing Co.: New Delhi, India, 2002; pp. 141–158. [Google Scholar]
- Piraino, S.; de Vito, D.; Schmich, J.; Bouillon, J.; Boero, F. Reverse development in Cnidaria. Can. J. Zool. 2004, 82, 1748–1754. [Google Scholar] [CrossRef]
- Larson, R.J. Water content, organic content, and carbon and nitrogen composition of medusae from the northeast Pacific. J. Exp. Mar. Biol. Ecol. 1986, 99, 107–120. [Google Scholar] [CrossRef]
- Lucas, C.H.; Pitt, K.A.; Purcell, J.E.; Lebrato, M.; Condon, R.H. What’s in a jellyfish? Proximate and elemental composition and biometric relationships for use in biogeochemical studies. Ecology 2011, 92, 1704. [Google Scholar] [CrossRef]
- Li, J.; Hsieh, Y.H.P. Traditional Chinese food technology and cuisine. Asia Pac. J. Clin. Nutr. 2004, 13, 147–155. [Google Scholar] [PubMed]
- Lucas, C.H. Biochemical composition of the mesopelagic coronate jellyfish Periphylla periphylla from the Gulf of Mexico. J. Mar. Biol. Assoc. UK 2009, 89, 77–81. [Google Scholar] [CrossRef]
- Omori, M.; Nakano, E. Jellyfish fisheries in southeast Asia. Hydrobiologia 2001, 451, 19–26. [Google Scholar] [CrossRef]
- Hsieh, Y.H.P.; Rudloe, J. Potential of utilizing jellyfish as food in Western countries. Trends Food Sci. Technol. 1994, 5, 225–229. [Google Scholar] [CrossRef]
- Hsieh, Y.H.P.; Leong, F.M.; Rudloe, J. Jellyfish as food. Hydrobiologia 2001, 451, 11–17. [Google Scholar] [CrossRef]
- Aouacheria, A.; Geourjon, C.; Aghajari, N.; Navratil, V.; Deléage, G.; Lethias, C.; Exposito, J.Y. Insights into early extracellular matrix evolution: Spongin short chain collagen-related proteins are homologous to basement membrane type IV collagens and form a novel family widely distributed in invertebrates. Mol. Biol. Evol. 2006, 23, 2288–2302. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Addad, S.; Exposito, J.Y.; Faye, C.; Ricard-Blum, S.; Lethias, C. Isolation, characterization and biological evaluation of jellyfish collagen for use in biomedical applications. Mar. Drugs 2011, 9, 967–983. [Google Scholar] [CrossRef] [PubMed]
- Meena, C.; Mengi, S.A.; Deshpande, S.G. Biomedical and industrial applications of collagen. Proc. Indian Acad. Sci. 1999, 111, 319–329. [Google Scholar]
- Exposito, J.Y.; Valcourt, U.; Cluzel, C.; Lethias, C. The fibrillar collagen family. Int. J. Mol. Sci. 2010, 11, 407–426. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Sun, L.; Zhao, X.; Wang, J.; Hou, H.; Li, B. Antioxidant and melanogenesis-inhibitory activities of collagen peptide from jellyfish (Rhopilema esculentum). J. Sci. Food Agric. 2009, 89, 1722–1727. [Google Scholar] [CrossRef]
- Zhuang, Y.; Hou, H.; Zhao, X.; Zhang, Z.; Li, B. Effects of collagen and collagen hydrolysate from jellyfish (Rhopilema esculentum) on mice skin photoaging induced by UV irradiation. J. Food Sci. 2009, 74, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, S.; Goto, Y.; Morishige, H.; Shiraishi, R.; Doi, M.; Akiyama, K.; Yamauchi, S.; Sugahara, T. Mode of action of the immunostimulatory effect of collagen from jellyfish. Biosci. Biotechnol. Biochem. 2008, 72, 2806–2814. [Google Scholar] [CrossRef] [PubMed]
- Morishige, H.; Sugahara, T.; Nishimoto, S.; Muranaka, A.; Ohno, F.; Shiraishi, R.; Doi, M. Immunostimulatory effects of collagen from jellyfish in vivo. Cytotechnology 2011, 63, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.H.P. Use of Jellyfish Collagen (type II) in the Treatment of Rheumatoid Arthritis. U.S. Patent 6,894,029 B1, 17 May 2005. [Google Scholar]
- Hoyer, B.; Bernhardt, A.; Lode, A.; Heinemann, S.; Sewing, J.; Klinger, M.; Notbohm, H.; Gelinsky, M. Jellyfish collagen scaffolds for cartilage tissue engineering. Acta Biomater. 2014, 10, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zhang, Z.; Pei, X.; Han, X.; Wang, J.; Wang, L.; Long, Z.; Shen, X.; Li, Y. Immunomodulatory effects of marine oligopeptide preparation from Chum Salmon (Oncorhynchus keta) in mice. Food Chem. 2009, 113, 464–470. [Google Scholar] [CrossRef]
- McCann, K.B.; Shiell, B.J.; Michalski, W.P.; Lee, A.; Wan, J.; Roginski, H.; Coventry, M.J. Isolation and characterisation of antibacterial peptides derived from the f(164-207) region of bovine alphaS2-casein. Int. Dairy J. 2005, 15, 133–143. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Kim, S.K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Hata, Y.; Yamamoto, M.; Ohni, M.; Nakajima, K.; Nakamura, Y.; Takano, T. A placebo-controlled study of the effect of sour milk on blood pressure in hypertensive subjects. Am. J. Clin. Nutr. 1996, 64, 767–771. [Google Scholar] [PubMed]
- Panyam, D. Enhancing the functionality of food proteins by enzymatic modification. Trends Food Sci. Technol. 1996, 7, 120–125. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, B.; Zhang, T.; Mu, W.; Liu, J. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH). Food Chem. 2008, 106, 444–450. [Google Scholar] [CrossRef]
- De Souza, L.M.; Iacomini, M.; Gorin, P.A.J.; Sari, R.S.; Haddad, M.A.; Sassaki, G.L. Glyco- and sphingophosphonolipids from the medusa Phyllorhiza punctata: NMR and ESI-MS/MS fingerprints. Chem. Phys. Lipids 2007, 145, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Dunn, S.R.; Thomas, M.C.; Nette, G.W.; Dove, S.G. A lipidomic approach to understanding free fatty acid lipogenesis derived from dissolved inorganic carbon within cnidarian-dinoflagellate symbiosis. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, Y.; Naganuma, T. Potential dietary effects on the fatty acid composition of the common jellyfish Aurelia aurita. Mar. Biol. 2001, 138, 1029–1035. [Google Scholar] [CrossRef]
- Papina, M.; Meziane, T.; van Woesik, R. Symbiotic zooxanthellae provide the host-coral Montipora digitata with polyunsaturated fatty acids. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2003, 135, 533–537. [Google Scholar] [CrossRef]
- Morris, R.J.; McCartney, M.J.; Schulze-Röbbecke, A. Bolinopsis infundibulum (O.F. Müller): Biochemical composition in relation to diet. J. Exp. Mar. Bio. Ecol. 1983, 67, 149–157. [Google Scholar] [CrossRef]
- Clarke, A. The lipid content and composition of some Antarctic macrozooplankton. Br. Antarct. Surv. Bull. 1984, 63, 57–70. [Google Scholar]
- Macrì, S. Nuove osservazioni intorno la storia naturale del polmone marino degli antichi; Biblioteca Regia Monacensis: Munich, Germany, 1778. (In Italian) [Google Scholar]
- Kogovšek, T.; Tinta, T.; Klun, K.; Malej, A. Jellyfish biochemical composition: Importance of standardised sample processing. Mar. Ecol. Prog. Ser. 2014, 510, 275–288. [Google Scholar] [CrossRef]
- Heaslip, S.G.; Iverson, S.J.; Bowen, W.D.; James, M.C. Jellyfish support high energy intake of leatherback sea turtles (Dermochelys coriacea): Video evidence from animal-borne cameras. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Milisenda, G.; Rosa, S.; Fuentes, V.L.; Boero, F.; Guglielmo, L.; Purcell, J.E.; Piraino, S. Jellyfish as prey: Frequency of predation and selective foraging of boops boops (vertebrata, actinopterygii) on the mauve stinger Pelagia noctiluca (Cnidaria, scyphozoa). PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- D’Ambra, I.; Graham, W.M.; Carmichael, R.H.; Hernandez, F.J. Fish rely on scyphozoan hosts as a primary food source: Evidence from stable isotope analysis. Mar. Biol. 2014, 162, 247–252. [Google Scholar] [CrossRef]
- Doyle, T.K.; Houghton, J.D.R.; McDevitt, R.; Davenport, J.; Hays, G.C. The energy density of jellyfish: Estimates from bomb-calorimetry and proximate-composition. J. Exp. Mar. Bio. Ecol. 2007, 343, 239–252. [Google Scholar] [CrossRef]
- Arai, M.N. A Functional Biology of Scyphozoa; Springer Science & Business Media: Dordrecht, The Netherlands, 1997. [Google Scholar]
- Food energy—Methods of analysis and conversion. In Report of a Technical Workshop; FAO: Rome, Italy, 2002; p. 93.
- Rolls, B.J.; Drewnowski, A.; Ledikwe, J.H. Changing the energy density of the diet as a strategy for weight management. J. Am. Diet. Assoc. 2005, 105, S98–S103. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.L.; Sun, L.P.; Zhao, X.; Hou, H.; Li, B.F. Investigation of gelatin polypeptides of jellyfish (Rhopilema esculentum) for their antioxidant activity in vitro. Food Technol. Biotechnol. 2010, 48, 222–228. [Google Scholar]
- Yu, H.; Li, R.; Liu, S.; Xing, R.; Chen, X.; Li, P. Amino acid composition and nutritional quality of gonad from jellyfish Rhopilema esculentum. Biomed. Prev. Nutr. 2014, 4, 399–402. [Google Scholar] [CrossRef]
- Krishnan, S.; Perumal, P. Preparation and biomedical characterization of jellyfish (Chrysaora Quinquecirrha) collagen from southeast coast of India. Int. J. Pharm. Pharm. Sci. 2013, 5, 698–701. [Google Scholar]
- Usydus, Z.; Szlinder-Richert, J.; Adamczyk, M. Protein quality and amino acid profiles of fish products available in Poland. Food Chem. 2009, 112, 139–145. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Guillen, M.C.; Gimenez, B.; Lopez-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Miura, S.; Kimura, S. Jellyfish mesogloea collagen. Characterization of molecules as alpha 1 alpha 2 alpha 3 heterotrimers. J. Biol. Chem. 1985, 260, 15352–15356. [Google Scholar] [PubMed]
- Nagai, T.; Ogawa, T.; Nakamura, T.; Ito, T.; Nakagawa, H.; Fujiki, K.; Nakao, M.; Yano, T. Collagen of edible jellyfish exumbrella. J. Sci. Food Agric. 1999, 79, 855–858. [Google Scholar] [CrossRef]
- Calejo, M.T.; Morais, Z.B.; Fernandes, A.I. Isolation and biochemical characterisation of a novel collagen from Catostylus tagi. J. Biomater. Sci. Polym. Ed. 2009, 20, 2073–2087. [Google Scholar] [CrossRef] [PubMed]
- Barzideh, Z.; Latiff, A.A.; Gan, C.Y.; Benjakul, S.; Karim, A.A. Isolation and characterisation of collagen from the ribbon jellyfish (Chrysaora sp.). Int. J. Food Sci. Technol. 2014, 49, 1490–1499. [Google Scholar] [CrossRef]
- Silva, T.; Moreira-Silva, J.; Marques, A.; Domingues, A.; Bayon, Y.; Reis, R. Marine origin collagens and its potential applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Duan, R.; Huang, L.; Song, Y.; Regenstein, J.M. Characterisation of acid-soluble and pepsin-solubilised collagen from jellyfish (Cyanea nozakii Kishinouye). Food Chem. 2014, 150, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Sewing, J.; Klinger, M.; Notbohm, H. Jellyfish collagen matrices conserve the chondrogenic phenotype in two- and three-dimensional collagen matrices. J. Tissue Eng. Regen. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Worawattanamateekul, W.; Suzuki, N.; Nakamura, T.; Ito, T.; Fujiki, K.; Nakao, M.; Yano, T. Isolation and characterization of collagen from rhizostomous jellyfish (Rhopilema asamushi). Food Chem. 2000, 70, 205–208. [Google Scholar] [CrossRef]
- Anal, A.K.; Noomhorm, A.; Vongsawasdi, P. Protein hydrolysates and bioactive peptides from seafood and crustacean waste: Their extraction, bioactive properties and industrial perspectives. Mar. Proteins Pept. Biol. Act. Appl. 2013, 709–735. [Google Scholar]
- Blanquet, R. Structural and chemical aspects of the podocyst cuticle of the scyphozoan medusa, Chrysaora quinquecirrha. Biol. Bull. 1972, 142, 1–10. [Google Scholar] [CrossRef]
- Mitchell, K. Organic Compounds in Cyanea capillata and Chrysaora quinquecirrha. Available online: http://digitalcommons.iwu.edu/cgi/viewcontent.cgi?article=1012&context=bio_honproj (accessed on 26 March 2014).
- Madhan, B.; Subramanian, V.; Rao, J.R.; Nair, B.U.; Ramasami, T. Stabilization of collagen using plant polyphenol: Role of catechin. Int. J. Biol. Macromol. 2005, 37, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.M.P.; Leme, A.A.; Aguiar, T.R.; Phansalkar, R.; Nam, J.W.; Bisson, J.; McAlpine, J.B.; Chen, S.N.; Pauli, G.F.; Bedran-Russo, A. Mimicking the hierarchical functions of dentin collagen cross-links with plant derived phenols and phenolic acids. Langmuir 2014, 30, 14887–14993. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Han, X.Q.; Synowiecki, J. Production and characteristics of protein hydrolysates from capelin (Mallotus villosus). Food Chem. 1995, 53, 285–293. [Google Scholar] [CrossRef]
- Kitts, D.D.; Weiler, K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Curr. Pharm. Des. 2003, 9, 1309–1323. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.C.; Chen, H.M.; Shiau, C.Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res. Int. 2003, 36, 949–957. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Kim, S.K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; de Lumen, B.O.; Hsieh, C.; Clemente, A.; Marín-manzano, C.; Arques, C.; Domoney, C.; Norris, R.; Fitzgerald, J.; Tavares, T.G.; et al. Bioactive Food Peptides in Health and Disease; Hernández-Ledesma, B., Hsieh, C.C., Eds.; InTech: Rijeka, Croatia, 2013. [Google Scholar]
- Kim, S.K. Marine Proteins and Peptides: Biological Activities and Applications; John Wiley & Sons, Ltd: Chichester, West Sussex, UK, 2013. [Google Scholar]
- Fan, J.; Zhuang, Y.; Li, B. Effects of collagen and collagen hydrolysate from jellyfish umbrella on histological and immunity changes of mice photoaging. Nutrients 2013, 5, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Sun, L.; Zhang, Y.; Liu, G. Antihypertensive effect of long-term oral administration of jellyfish (Rhopilema esculentum) collagen peptides on renovascular hypertension. Mar. Drugs 2012, 10, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Barzideh, Z.; Latiff, A.A.; Gan, C.; Alias, A.K. ACE inhibitory and antioxidant activities of collagen hydrolysates from the ribbon jellyfish (Chrysaora sp.). Food Technol. Biotecnol. 2014, 52, 495–504. [Google Scholar] [CrossRef]
- Leblond, J.D.; Chapman, P.J. Lipid class distribution of highly unsaturated long chain fatty acids in marine dinoflagellates. J. Phycol. 2000, 36, 1103–1108. [Google Scholar] [CrossRef]
- Halver, J.E. Chapter 4: Lipids and fatty acids. In ADCP/REP/80/11—Fish Feed Technology, United Nations Development Programme, Food and Agriculture Organization of the United Nations; FAO: Rome, Italy, 1980; pp. 41–53. [Google Scholar]
- Russo, G.L. Dietary n-6 and n-3 polyunsaturated fatty acids: From biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharmacol. 2009, 77, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Siriwardhana, N.; Kalupahana, N.S.; Moustaid-Moussa, N. Health benefits of n-3 polyunsaturated fatty acids: Eicosapentaenoic acid and docosahexaenoic acid. Adv. Food Nutr. Res. 2012, 65, 211–222. [Google Scholar] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Bond, M.D.; Van Wart, H.E. Purification and separation of individual collagenases of Clostridium histolyticum using red dye ligand chromatography. Biochem. 1984, 23, 3077–3085. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Longo, C.; Leo, L.; Leone, A. Carotenoids, fatty acid composition and heat stability of supercritical carbon dioxide-extracted-oleoresins. Int. J. Mol. Sci. 2012, 13, 4233–4254. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Szczęsna-Antczak, M.; Antczak, T.; Piotrowicz-Wasiak, M.; Rzyska, M.; Binkowska, N.; Bielecki, S. Relationships between lipases and lipids in mycelia of two Mucor strains. Enzyme Microb. Technol. 2006, 39, 1214–1222. [Google Scholar] [CrossRef]
- Talà, A.; Lenucci, M.S.; Gaballo, A.; Durante, M.; Tredici, S.M.; Debowles, D.A.; Pizzolante, G.; Marcuccio, C.; Carata, E.; Piro, G.; et al. Sphingomonas cynarae sp. nov., a proteobacterium that produces an unusual type of sphingan. Int. J. Syst. Evol. Microbiol. 2013, 63, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Querellou, J.; Børresen, T.; Boyen, C.; Dobson, A.; Höfle, M.; Ianora, A.; Jaspars, M.; Kijjoa, A.; Olafsen, J.; Rigos, G.; Wijffels, R.H. Marine Biotechnology: A New Vision and Strategy for Europe; Marine Board, Ed.; European Science Foundation: Beernem, Belgium, 2010. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leone, A.; Lecci, R.M.; Durante, M.; Meli, F.; Piraino, S. The Bright Side of Gelatinous Blooms: Nutraceutical Value and Antioxidant Properties of Three Mediterranean Jellyfish (Scyphozoa). Mar. Drugs 2015, 13, 4654-4681. https://doi.org/10.3390/md13084654
Leone A, Lecci RM, Durante M, Meli F, Piraino S. The Bright Side of Gelatinous Blooms: Nutraceutical Value and Antioxidant Properties of Three Mediterranean Jellyfish (Scyphozoa). Marine Drugs. 2015; 13(8):4654-4681. https://doi.org/10.3390/md13084654
Chicago/Turabian StyleLeone, Antonella, Raffaella Marina Lecci, Miriana Durante, Federica Meli, and Stefano Piraino. 2015. "The Bright Side of Gelatinous Blooms: Nutraceutical Value and Antioxidant Properties of Three Mediterranean Jellyfish (Scyphozoa)" Marine Drugs 13, no. 8: 4654-4681. https://doi.org/10.3390/md13084654
APA StyleLeone, A., Lecci, R. M., Durante, M., Meli, F., & Piraino, S. (2015). The Bright Side of Gelatinous Blooms: Nutraceutical Value and Antioxidant Properties of Three Mediterranean Jellyfish (Scyphozoa). Marine Drugs, 13(8), 4654-4681. https://doi.org/10.3390/md13084654