Development and Application of a Novel SPE-Method for Bioassay-Guided Fractionation of Marine Extracts
Abstract
:1. Introduction
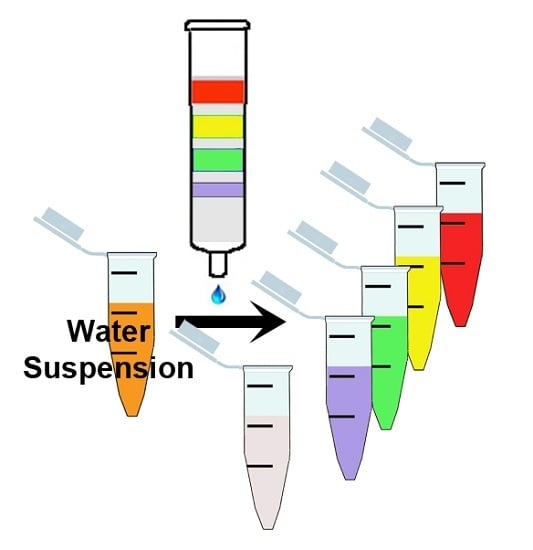
2. Results and Discussion
| Phylum | Sample Name | Main Secondary Component | Ref. | |
|---|---|---|---|---|
| Porifera | Dendrillamembranosa | 9,11-dihydrogracilin A |  1 1 | [16] |
| Porifera | Renierasarai | sarains A–C |  e.g., sarain A (2) e.g., sarain A (2) | [17,18] |
| sarains 1–3 |  e.g., sarain 1 (3) e.g., sarain 1 (3) | |||
| Dinoflagellata | Amphidiniumcarterae (CCMP121) |  | [19] | |
| amphidinol-18amphidinol-19 | 4 R = H 5 R = SO3− | |||

| (1) Sample Preparation | (2) Column Equilibration | (3) Elution Gradient: |
|---|---|---|
|
| W—Washing step 100% H2O (2 mL);
|




3. Experimental Section
3.1. General
3.2. Algal Culturing
3.3. Extraction and Fractionation
3.4. Isolation and Characterization of Lyso-PAF Analogs from Antarctic Sponges
3.5. Antibacterial Test
3.6. Immunostimulatory Test
3.7. Statistical Analysis
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2015, 32, 116–211, and previous reviews of the series. [Google Scholar] [CrossRef] [PubMed]
- Mora, C.; Tittersor, D.P.; Adl, S.; Simpson, A.G.B.; Worm, B. How Many Species Are There on Earth and in the Ocean? PLoS Biol. 2011, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Giddings, L.A. Natural products as leads to antitumor drugs. Phytochem. Rev. 2014, 13, 123–137. [Google Scholar] [CrossRef]
- Duarte, K.; Justino, C.I.L.; Comes, A.M.; Rocha-Santos, T.; Duarte, A.C. Green Analytical Methodologies for Preparation of Extracts and Analysis of Bioactive Compounds. In Comprehensive Analytical Chemistry: Analysis of Marine Samples in Search of Bioactive Compounds; Rocha-Santos, T., Duarte, A.C., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 65, pp. 59–78. [Google Scholar]
- Moldoveanu, S.; David, V. Solid phase extraction. In Modern Sample Preparation for Chromatography; Moldoveanu, S., David, V., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 191–286. [Google Scholar]
- Majors, R.E. Solid Phase Extraction. In Handobook of Sample Preparations; Pawliszyn, J., Lord, H.L., Eds.; Wiley Sons: Hoboken, NJ, USA, 2010; pp. 53–79. [Google Scholar]
- Lundanes, E.; Reubsaet, L.; Greibrokk, T. Chromatography: Basic Principles, Sample Preparations and Related Methods, 1st ed.; Wiley-VCH Verlag: Weinheim, Germany, 2014. [Google Scholar]
- Huck, C.W.; Bonn, G.W. Poly(styrene-divynylbenzene) based media for liquid chromatography. Chem. Eng. Technol. 2005, 28, 1457–1472. [Google Scholar] [CrossRef]
- Chambers, T.K. Liquid chromatographic retention studies using polystyrene-divinylbenzene stationary phases in reversed-phase and normal-phase eluents. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 1996. [Google Scholar]
- Minale, L.; Pizza, C.; Riccio, R.; Zollo, F. Steroidalglycosides from starfishes. Pure Appl. Chem. 1982, 54, 1935–1950. [Google Scholar] [CrossRef]
- West, L.M.; Northcote, P.T. Peloruside A: A Potent Cytotoxic Macrolide Isolated from the New Zealand Marine Sponge Mycale sp. J. Org. Chem. 2000, 65, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Houssen, E.W.; Jaspars, M. Isolation of marine natural products. In Methods in Biotechnology; Satyajit, D., Sarker, S.D., Zahid Latif, Z., Alexander, I., Gray, A.I., Eds.; Humana Press: Totowa, NJ, USA, 2005; Volume 20, pp. 353–390. [Google Scholar]
- West, L.W.; Faulkner, D.J. Hexaprenoid Hydroquinones from the Sponge Haliclona (aka Adocia) sp. J. Nat. Prod. 2006, 69, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Bugni, T.S.; Harper, M.K.; McCulloch, M.W.B.; Reppart, J.; Ireland, C.M. Fractionated Marine Invertebrate Extract Libraries for Drug Discovery. Molecules 2008, 13, 1372–1383. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Zhou, Y.-D.; Nagle, D.G. Comparative Study of Chromatographic Medium-Associated Mass and Potential Antitumor Activity Loss with Bioactive Extracts. J. Nat. Prod. 2013, 76, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Molinski, T.F.; Faulkner, D.J. Metabolites of the Antarctic Sponge Dendrillamembranosa. J. Org. Chem. 1987, 52, 298–300. [Google Scholar] [CrossRef]
- Guo, Y.; Madaio, A.; Trivellone, E.; Scognamiglio, G.; Cimino, G. Further Studies of Alkaloids from Renierasarai: Structures of Saraine-3 and Isosaraine-3; Absolute Stereochemistry of Saraine-1 and Saraine-2. Tetrahedron 1996, 52, 14961–14974. [Google Scholar] [CrossRef]
- Guo, Y.; Madaio, A.; Trivellone, E.; Scognamiglio, G.; Cimino, G. Structural and Stereochemical Studies of Saraines: Macrocyclic Alkaloids of the Sponge Renierasarai. Tetrahedron 1996, 52, 8341–8348. [Google Scholar] [CrossRef]
- Nuzzo, G.; Cutignano, A.; Sardo, A.; Fontana, A. Antifungal Amphidinol 18 and Its 7-Sulfate Derivative from the Marine Dinoflagellate Amphidinium carterae. J. Nat. Prod. 2014, 77, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.J.; Lee, Y.K. Determination of biomass dry weight of marine microalgae. J. Appl. Phycol. 1997, 9, 189–194. [Google Scholar] [CrossRef]
- De Gregorio, E. The path forward. Vaccines 2015, 33S, B60–B63. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Y.; Singh, M.; Miller, A.T.; de Gregorio, E.; Doro, F.; D’Oro, U.; Skibinski, D.A.; Mbow, M.L.; Bufali, S.; et al. Rational design of small molecules as vaccine adjuvants. Sci. Transl. Med. 2014, 6. [Google Scholar] [CrossRef] [PubMed]
- Deering, R.D.; Orange, J.S. Development of a clinical assay to evaluate Toll-like receptor function. Clin. Vaccine Immunol. 2006, 13, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Espiritu, R.A.; Matsumori, N.; Tsuda, M.; Murata, M. Direct and stereospecific interaction of Amphidinol 3 with sterol in lipid bilayers. Biochemistry 2014, 53, 3287–3293. [Google Scholar] [CrossRef] [PubMed]
- Houdai, T.; Matsuoka, S.; Morsy, N.; Matsumori, N.; Satake, M.; Murata, M. Hairpin conformation of amphidinols possibly accounting for potent membrane permeabilizing activities. Tetrahedron 2005, 61, 2795–2802. [Google Scholar] [CrossRef]
- Muller, W.E.G.; Klemt, M.; Thakur, N.L.; Schroder, C.; Aiello, A.; D’Esposito, M.; Menna, M.; Fattorusso, E. Molecular/chemical ecology in sponges: Evidence for an adaptive antibacterial response in Suberites domuncula. Mar. Biol. 2004, 144, 19–29. [Google Scholar] [CrossRef]
- Shin, B.A.; Kim, Y.R.; Lee, I.S.; Sung, C.K.; Hong, J.; Sim, C.; Im, K.S.; Jung, J.H. Lyso-PAF analogues and lysophosphatidylcholines from the marine sponge Spirastrella abata as inhibitors of cholesterol biosynthesis. J. Nat. Prod. 1999, 62, 1554–1557. [Google Scholar] [CrossRef] [PubMed]
- Alam, N.; Bae, B.H.; Hong, J.; Lee, C.O.; Shin, B.A.; Im, K.S.; Jung, J.H. Additional bioactive lyso-PAF congeners from the sponge Spirastrella abata. J. Nat. Prod. 2001, 64, 533–535. [Google Scholar] [CrossRef] [PubMed]
- Steel, H.C.; Cockeran, R.; Anderson, A. Platelet-activating factor and lyso-PAF possess direct antimicrobial properties in vitro. APMIS 2002, 110, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Cho, K.; Kim, Y.H.; Cheong, C.; Lee, K.S.; Jung, J.H. Structural determination of lysophosphatidylcholines extracted from marine sponges by fast atom bombardment tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2001, 15, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Keller, D.K.; Selvin, R.C.; Claus, W.; Guillard, R.R.L. Media for the culture of oceanic ultraphytoplankton. J. Phycol. 1987, 23, 633–638. [Google Scholar] [CrossRef]
- Guillard, R.R.L. Culture of phytoplankton for feeding marine invertebrates. In Culture of Marine Invertebrate Animals; Smith, W.L., Ed.; Plenum Press: New York, NY, USA, 1975; pp. 29–60. [Google Scholar]
- Gerwick, W.H.; Moore, B.S. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem. Biol. 2012, 19, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine pharmacology in 2009–2011: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of action. Mar. Drugs 2013, 11, 2510–2573. [Google Scholar] [PubMed]
- Santos, E.A.; Quintela, A.L.; Ferreira, E.G.; Sousa, T.S.; Pinto, F.; Hajdu, E.; Carvalho, M.S.; Salani, S.; Rocha, D.D.; Wilke, D.V.; et al. Cytotoxic Plakortides from the Brazilian Marine Sponge Plakortis angulospiculatus. J. Nat. Prod. 2015, 78, 996–1004. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cutignano, A.; Nuzzo, G.; Ianora, A.; Luongo, E.; Romano, G.; Gallo, C.; Sansone, C.; Aprea, S.; Mancini, F.; D'Oro, U.; et al. Development and Application of a Novel SPE-Method for Bioassay-Guided Fractionation of Marine Extracts. Mar. Drugs 2015, 13, 5736-5749. https://doi.org/10.3390/md13095736
Cutignano A, Nuzzo G, Ianora A, Luongo E, Romano G, Gallo C, Sansone C, Aprea S, Mancini F, D'Oro U, et al. Development and Application of a Novel SPE-Method for Bioassay-Guided Fractionation of Marine Extracts. Marine Drugs. 2015; 13(9):5736-5749. https://doi.org/10.3390/md13095736
Chicago/Turabian StyleCutignano, Adele, Genoveffa Nuzzo, Adrianna Ianora, Elvira Luongo, Giovanna Romano, Carmela Gallo, Clementina Sansone, Susanna Aprea, Francesca Mancini, Ugo D'Oro, and et al. 2015. "Development and Application of a Novel SPE-Method for Bioassay-Guided Fractionation of Marine Extracts" Marine Drugs 13, no. 9: 5736-5749. https://doi.org/10.3390/md13095736
APA StyleCutignano, A., Nuzzo, G., Ianora, A., Luongo, E., Romano, G., Gallo, C., Sansone, C., Aprea, S., Mancini, F., D'Oro, U., & Fontana, A. (2015). Development and Application of a Novel SPE-Method for Bioassay-Guided Fractionation of Marine Extracts. Marine Drugs, 13(9), 5736-5749. https://doi.org/10.3390/md13095736