Bioresponsive Materials for Drug Delivery Based on Carboxymethyl Chitosan/Poly(γ-Glutamic Acid) Composite Microparticles
Abstract
:1. Introduction
2. Results and Discussion
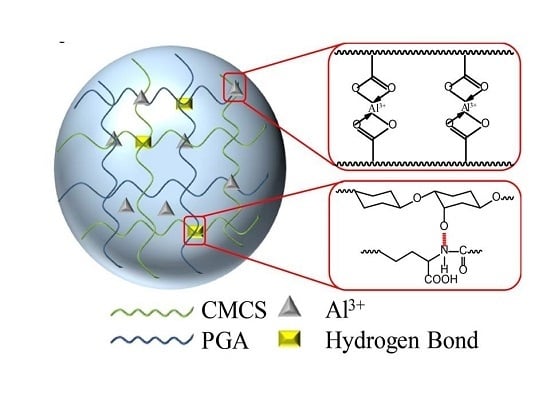
2.1. Characterization of CMCS/PGA Composite Microparticles
2.2. Morphology of CMCS/PGA Composite Microparticles
2.3. Swelling Behaviors of CMCS/PGA Composite Microparticles
2.4. Hydrophilic Properties of CMCS/PGA Composite Microparticles
2.5. Drug Release Behaviors of CMCS/PGA Composite Microparticles
3. Materials and Methods
3.1. Materials
3.2. Preparetion of CMCS/PGA Composite Microparticles
3.3. Characterization of CMCS/PGA Composite Microparticles
3.3.1. Fourier Transform Infrared Spectroscopy (FTIR) Analyses
3.3.2. XRD Diffractograms Analyses
3.3.3. X-ray Photoelectron Spectroscopy (XPS) Analyses
3.3.4. Surface Analysis via Scanning Electron Microscopy
3.4. Determination of the Swelling Behavior of Composite Microparticles
3.5. Water Contact Angle Measurement
3.6. Pharmacokinetic Drug Release Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Allen, T.M.; Cullis, P.R. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Anil, S.; Kim, S.; Shim, M.S. Seaweed Polysaccharide-Based Nanoparticles: Preparation and Applications for Drug Delivery. Polymers 2016, 8, 30. [Google Scholar] [CrossRef]
- Pakulska, M.M.; Miersch, S.; Shoichet, M.S. Designer protein delivery: From natural to engineered affinity-controlled release systems. Science 2016, 351, 1279. [Google Scholar] [CrossRef] [PubMed]
- Fox, C.B.; Kim, J.; Le, L.V.; Nemeth, C.L.; Chirra, H.D.; Desai, T.A. Micro/nanofabricated platforms for oral drug delivery. J. Control. Release 2015, 219, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Skorb, E.V.; Möhwald, H.; Andreeva, D.V. How Can One Controllably Use of Natural ΔpH in Polyelectrolyte Multilayers? Adv. Mater. Interfaces 2017, 4, 1600282. [Google Scholar] [CrossRef]
- Corobea, M.C.; Muhulet, O.; Miculescu, F.; Antoniac, I.V.; Vuluga, Z.; Florea, D.; Vuluga, D.M.; Butnaru, M.; Ivanov, D.; Voicu, S.I.; et al. Novel nanocomposite membranes from cellulose acetate and clay-silica nanowires. Polym. Adv. Technol. 2016, 27, 1586–1595. [Google Scholar] [CrossRef]
- Voicu, S.I.; Condruz, R.M.; Mitran, V.; Cimpean, A.; Miculescu, F.; Andronescu, C.; Miculescu, M.; Thakur, V.K. Sericin Covalent Immobilization onto Cellulose Acetate Membrane for Biomedical Applications. ACS Sustain. Chem. Eng. 2016, 4, 1765–1774. [Google Scholar] [CrossRef]
- Thakur, M.K.; Thakur, V.K.; Gupta, R.K.; Pappu, A. Synthesis and Applications of Biodegradable Soy Based Graft Copolymers: A Review. ACS Sustain. Chem. Eng. 2016, 4, 1–17. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Recent Advances in Graft Copolymerization and Applications of Chitosan: A Review. ACS Sustain. Chem. Eng. 2014, 2, 2637–2652. [Google Scholar] [CrossRef]
- Casettari, L.; Illum, L. Chitosan in nasal delivery systems for therapeutic drugs. J. Control. Release 2014, 190, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesaro, A. “The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Ding, D.; Ren, J.; Zhu, X.; Yao, Y. Synthesis, characterization, and drug delivery property of 2-N-carboxymethyl-6-O-diethylaminoethyl-chitosan. e-Polymers 2013, 13. [Google Scholar] [CrossRef]
- Chen, Y.; Yi, J.; Gao, Q.; Zhou, X.; Luo, Y.; Liu, P. Surface Performance and Cytocompatibility Evaluation of Acrylic Acid-Mediated Carboxymethyl Chitosan Coating on Poly(tetrafluoroethylene-co-hexafluoropropylene). Plasma Chem. Plasma Process. 2013, 33, 1153–1165. [Google Scholar] [CrossRef]
- Sereshti, H.; Samadi, S.; Asgari, S.; Karimi, M. Preparation and application of magnetic graphene oxide coated with a modified chitosan pH-sensitive hydrogel: An efficient biocompatible adsorbent for catechin. RSC Adv. 2015, 5, 9396–9404. [Google Scholar] [CrossRef]
- De Kruif, J.K.; Fasler-Kan, E.; Varum, F.; Bravo, R.; Kuentz, M. On Prilling of Hydrophilic Microgels in Lipid Dispersions Using Mono-N-Carboxymethyl Chitosan for Oral Biologicals Delivery. J. Pharm. Sci. 2014, 103, 3675–3687. [Google Scholar] [CrossRef] [PubMed]
- Samanta, H.S.; Ray, S.K. Controlled release of tinidazole and theophylline from chitosan based composite hydrogels. Carbohydr. Polym. 2014, 106, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zuo, Q.; Guo, R.; Hong, A.; Li, C.; Zhang, Y.; He, L.; Xue, W. Fabrication and characterization of carboxymethyl chitosan/poly(vinyl alcohol) hydrogels containing alginate microspheres for protein delivery. J. Bioact. Compat. Polym. 2015, 30, 397–411. [Google Scholar] [CrossRef]
- Chen, M.; Ling, M.; Kusuma, S.J. Poly-γ-glutamic acid microneedles with a supporting structure design as a potential tool for transdermal delivery of insulin. Acta Biomater. 2015, 24, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Tang, Z.; Zhang, D.; Song, W.; Zhanga, Y.; Yang, Y.; Ahmad, Z.; Chen, X. Pharmacokinetics, biodistribution and in vivo efficacy of cisplatin loaded poly(l-glutamic acid)-g-methoxy poly(ethylene glycol) complex nanoparticles for tumor therapy. J. Control. Release 2015, 205, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Conejos-Sanchez, I.; Cardoso, I.; Oteo-Vives, M.; Romero-Sanz, E.; Paul, A.; Ruiz Sauri, A.; Morcillo, M.A.; Saraiva, M.J.; Vicent, M.J. Polymer-doxycycline conjugates as fibril disrupters: An approach towards the treatment of a rare amyloidotic disease. J. Control. Release 2015, 198, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Xu, M.; Cao, Z.; Gao, J.; Chen, Y.; Li, Y.; Yang, Z.; Xie, X.; Jiang, Q.; Wang, W.; et al. Ultrasound-Triggered Phase-Transition Cationic Nanodroplets for Enhanced Gene Delivery. ACS Appl. Mater. Interfaces 2015, 7, 13524–13537. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Lin, C.; Hsu, C.; Ho, Y.; Chuang, E.; Sung, H.; Mi, F. FRET-Based Dual-Emission and pH-Responsive Nanocarriers for Enhanced Delivery of Protein Across Intestinal Epithelial Cell Barrier. ACS Appl. Mater. Interfaces 2014, 6, 18275–18289. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Peng, S.; Chiu, Y.; Hsiao, C.; Liu, H.; Lim, W.; Lu, H.; Sung, H. Enhancement of efficiency of chitosan-based complexes for gene transfection with poly(γ-glutamic acid) by augmenting their cellular uptake and intracellular unpackage. J. Control. Release 2014, 193, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Yu, S.; Chao, A.; Wu, J.; Lin, Y.; Lu, K.; Mi, F. Preparation and properties of pH-responsive, self-assembled colloidal nanoparticles from guanidine-containing polypeptide and chitosan for antibiotic delivery. Colloids Surf. A Physicochem. Eng. Asp. 2016, 494, 9–20. [Google Scholar] [CrossRef]
- Teixeira, G.Q.; Pereira, C.L.; Castro, F.; Ferreira, J.R.; Gomez-Lazaro, M.; Aguiar, P.; Barbosa, M.A.; Neidlinger-Wilke, C.; Goncalves, R.M. Anti-inflammatory Chitosan/Poly-γ-glutamic acid nanoparticles control inflammation while remodeling extracellular matrix in degenerated intervertebral disc. Acta Biomater. 2016, 42, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Lee, J.; Lee, H.G. Nanoencapsulation of Red Ginseng Extracts Using Chitosan with Polyglutamic Acid or Fucoidan for Improving Antithrombotic Activities. J. Agric. Food Chem. 2016, 64, 4765–4771. [Google Scholar] [CrossRef] [PubMed]
- Park, B.G.; Kang, H.; Lee, W.; Kim, J.S.; Son, T. Reinforcement of pH-responsive γ-poly(glutamic acid)/chitosan hydrogel for orally administrable colon-targeted drug delivery. J. Appl. Polym. Sci. 2013, 127, 832–836. [Google Scholar] [CrossRef]
- Yan, S.; Rao, S.; Zhu, J.; Wang, Z.; Zhang, Y.; Duan, Y.; Chen, X.; Yin, J. Nanoporous multilayer poly(l-glutamic acid)/chitosan microcapsules for drug delivery. Int. J. Pharm. 2012, 427, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wu, S.; Tang, D.; Ho, Y.; Mi, F.; Kuo, T.; Sung, H. Stimuli-responsive materials prepared from carboxymethyl chitosan and poly(γ-glutamic acid) for protein delivery. Carbohydr. Polym. 2012, 87, 531–536. [Google Scholar] [CrossRef]
- Ko, J.A.; Park, H.J.; Hwang, S.J.; Park, J.B.; Lee, J.S. Preparation and characterization of chitosan microparticles intended for controlled drug delivery. Int. J. Pharm. 2002, 249, 165–174. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Strand, S.; Vårum, K.; Draget, K.; Nordgård, C. Chitosan: Gels and Interfacial Properties. Polymers 2015, 7, 552–579. [Google Scholar] [CrossRef]
- Lapitsky, Y. Ionically crosslinked polyelectrolyte nanocarriers: Recent advances and open problems. Curr. Opin. Colloid 2014, 19, 122–130. [Google Scholar] [CrossRef]
- Bao, D.; Chen, M.; Wang, H.; Wang, J.; Liu, C.; Sun, R. Preparation and characterization of double crosslinked hydrogel films from carboxymethylchitosan and carboxymethylcellulose. Carbohydr. Polym. 2014, 110, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Lin, C.; Ho, G.; Huang, Y.; Lee, Y. Synthesis and self-assembly of comb-like amphiphilic Doxifluridine-poly(epsilon-caprolactone)-graft-poly(γ-glutamic acid) copolymer. Polymer 2009, 50, 1755–1763. [Google Scholar] [CrossRef]
- Tang, H.; Chen, Y.; Tan, H. Primary study on preparation and water absorption property of the CMCTS-g-(PAA-co-PDMDAAC) polyampholytic superabsorbent polymer. Polym. Mater. Sci. Eng. 2006, 22, 115–118. [Google Scholar]
- Fang, J.; Zhang, Y.; Yan, S.; Liu, Z.; He, S.; Cui, L.; Yin, J. Poly(l-glutamic acid)/chitosan polyelectrolyte complex porous microspheres as cell microcarriers for cartilage regeneration. Acta Biomater. 2014, 10, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Shen, Y.; Shen, C.; Wen, Y.; Liu, W. Al-doping chitosan-Fe(III) hydrogel for the removal of fluoride from aqueous solutions. Chem. Eng. J. 2014, 248, 98–106. [Google Scholar] [CrossRef]
- Ng, M.; Liana, A.E.; Liu, S.; Lim, M.; Chow, C.W.K.; Wang, D.; Drikas, M.; Amal, R. Preparation and characterisation of new-polyaluminum chloride-chitosan composite coagulant. Water Res. 2012, 46, 4614–4620. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liang, Y.; Chen, N.; Guo, Y.; Ye, Y.; Tan, H. A Sustained Release of Model Drug from a Novel Polyacrylic Acid-polyaluminium Chloride Superabsorbent. Iran. Polym. J. 2010, 19, 531–540. [Google Scholar]
- Chen, Y.; Tan, H.M. Crosslinked carboxymethylchitosan-g-poly(acrylic acid) copolymer as a novel superabsorbent polymer. Carbohydr. Res. 2006, 341, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Ranjha, N.M.; Shahzad, Y. Swelling and controlled release of tramadol hydrochloride from a pH-sensitive hydrogel. Des. Monomers Polym. 2011, 14, 233–249. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, V. Influence of polymer network parameters of tragacanth gum-based pH responsive hydrogels on drug delivery. Carbohydr. Polym. 2014, 101, 928–940. [Google Scholar] [CrossRef] [PubMed]






| mCMCS:mPGA | σ0/E (g·g−1) | τ0 (min) | ki (g·min−1) | tc (min) | |
|---|---|---|---|---|---|
| CMCS | 5.105 | 7.712 | 0.383 | 9.394 | |
| 9:1 | 9.316 | 7.333 | 0.740 | 8.788 | |
| 8:2 | 10.738 | 6.355 | 0.987 | 7.576 | |
| 7:3 | 8.006 | 7.308 | 0.637 | 8.788 | |
| 6:4 | 6.462 | 7.897 | 0.478 | 9.394 | |
| CCMCS+PGA (g/mL) | σ0/E (g·g−1) | τ0 (min) | ki (g·min−1) | tc (min) | |
| 0.008 | 4.786 | 6.513 | 0.426 | 7.879 | |
| 0.010 | 7.690 | 6.338 | 0.708 | 7.576 | |
| 0.020 | 8.902 | 5.355 | 0.974 | 6.354 | |
| 0.024 | 5.763 | 5.589 | 0.602 | 6.667 | |
| 0.032 | 4.985 | 6.239 | 0.463 | 7.576 | |
| Conditions | Model | Fitting Equation | R |
|---|---|---|---|
| CAl = 0.05 μg/mL | Zero-order equation | y = 0.0019t + 0.3971 | 0.7092 |
| One-order equation | ln(1 − y) = −0.0043t − 0.5346 | 0.8089 | |
| Higuchi equation | y = 0.0389t1/2 + 0.2349 | 0.8192 | |
| CAl = 0.10 μg/mL | Zero-order equation | y = 0.0020t + 0.3203 | 0.8292 |
| One-order equation | ln(1 − y) = −0.0044t − 0.3810 | 0.9072 | |
| Higuchi equation | y = 0.0413t1/2 + 0.1535 | 0.9142 | |
| CAl = 0.20 μg/mL | Zero-order equation | y = 0.0019t + 0.2706 | 0.8613 |
| One-order equation | ln(1 − y)= −0.0037t − 0.3038 | 0.9304 | |
| Higuchi equation | y = 0.0388t1/2 + 0.1167 | 0.9317 |
| Samples | mCMCS:mPGA | Polymer Concentration (mg/mL) | [Al3+] (g/mL) |
|---|---|---|---|
| 1 | 10:0 | 20 | 0.1 |
| 2 | 9:1 | 22 | |
| 3 | 8:2 | 25 | |
| 4 | 7:3 | 29 | |
| 5 | 6:4 | 33 | |
| 6 | 8:2 | 10 | 0.1 |
| 7 | 12.5 | ||
| 8 | 25 | ||
| 9 | 30 | ||
| 10 | 40 | ||
| 11 | 8:2 | 25 | 0.05 |
| 12 | 0.1 | ||
| 13 | 0.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Tong, Z.; Chen, Y.; Mo, Y.; Feng, H.; Li, P.; Qu, X.; Jin, S. Bioresponsive Materials for Drug Delivery Based on Carboxymethyl Chitosan/Poly(γ-Glutamic Acid) Composite Microparticles. Mar. Drugs 2017, 15, 127. https://doi.org/10.3390/md15050127
Yan X, Tong Z, Chen Y, Mo Y, Feng H, Li P, Qu X, Jin S. Bioresponsive Materials for Drug Delivery Based on Carboxymethyl Chitosan/Poly(γ-Glutamic Acid) Composite Microparticles. Marine Drugs. 2017; 15(5):127. https://doi.org/10.3390/md15050127
Chicago/Turabian StyleYan, Xiaoting, Zongrui Tong, Yu Chen, Yanghe Mo, Huaiyu Feng, Peng Li, Xiaosai Qu, and Shaohua Jin. 2017. "Bioresponsive Materials for Drug Delivery Based on Carboxymethyl Chitosan/Poly(γ-Glutamic Acid) Composite Microparticles" Marine Drugs 15, no. 5: 127. https://doi.org/10.3390/md15050127
APA StyleYan, X., Tong, Z., Chen, Y., Mo, Y., Feng, H., Li, P., Qu, X., & Jin, S. (2017). Bioresponsive Materials for Drug Delivery Based on Carboxymethyl Chitosan/Poly(γ-Glutamic Acid) Composite Microparticles. Marine Drugs, 15(5), 127. https://doi.org/10.3390/md15050127