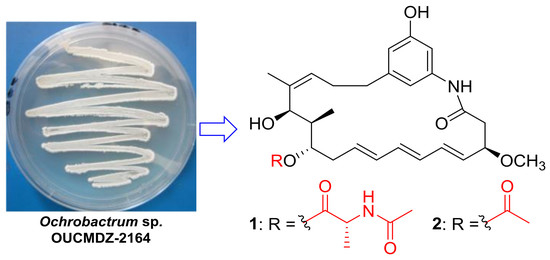
New Ansamycins from the Deep-Sea-Derived Bacterium Ochrobactrum sp. OUCMDZ-2164
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Collection and Phylogenetic Analysis
3.3. Cultivation and Extraction
3.4. Purification
3.5. Determination of the Absolute Configurations of 1 and 2
3.6. Cytotoxic Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, X.; Zhu, J.; Shi, G.; Sun, M.; Guo, Z.; Wang, H.; Lu, C.; Shen, Y. Deletion of the side chain assembly reveals diverse post-PKS modifications in the biosynthesis of ansatrienins. RSC Adv. 2016, 6, 88571–88579. [Google Scholar] [CrossRef]
- Xu, W.; Pang, K.-L.; Luo, Z.-H. High fungal diversity and abundance recovered in the deep-sea sediments of the Pacific Ocean. Microb. Ecol. 2014, 68, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-F.; Menna, M.; Cai, Y.-S.; Guo, Y.-W. Polyacetylenes of marine origin: Chemistry and bioactivity. Chem. Rev. 2015, 115, 1543–1596. [Google Scholar] [CrossRef] [PubMed]
- Bernan, V.S.; Greenstein, M.; Maiese, W.M. Marine microorganisms as a source of new natural products. Adv. Appl. Microbiol. 1997, 43, 57–90. [Google Scholar] [PubMed]
- Ward, A.C.; Bora, N. Diversity and biogeography of marine actinobacteria. Curr. Opin. Microbiol. 2006, 9, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Fenical, W.; Jensen, P.R. Developing a new resource for drug discovery: Marine actinomycete bacteria. Nat. Chem. Biol. 2006, 2, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhu, T.; Zhu, W. New marine natural products of microbial origin from 2010 to 2013. Chin. J. Org. Chem. 2013, 33, 1195–1234. [Google Scholar] [CrossRef]
- Zhu, T.-H.; Ma, Y.-N.; Wang, W.-L.; Chen, Z.-B.; Qin, S.-D.; Du, Y.-Q.; Wang, D.-Y.; Zhu, W.-M. New marine natural products from the marine-derived fungi other than Penicillum sp. and Aspergillus sp. (1951–2014). Chin. J. Mar. Drugs 2015, 34, 56–108. [Google Scholar]
- Ma, H.-G.; Liu, Q.; Zhu, G.-L.; Liu, H.-S.; Zhu, W.-M. Marine natural products sourced from marine-derived Penicillium fungi. J. Asian Nat. Prod. Res. 2016, 18, 92–115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Liu, H.; Zhu, W. New natural products from the marine-derived Aspergillus fungi-A review. Acta Microbiol. Sin. 2015, 56, 331–362. [Google Scholar]
- Schinke, C.; Martins, T.; Queiroz, S.C.N.; Melo, I.S.; Reyes, F.G.R. Antibacterial compounds from marine bacteria, 2010–2015. J. Nat. Prod. 2017, 80, 1215–1228. [Google Scholar] [CrossRef] [PubMed]
- Pathom-aree, W.; Stach, J.E.; Ward, A.C.; Horikoshi, K.; Bull, A.T.; Goodfellow, M. Diversity of actinomycetes isolated from challenger deep sediment (10,898 m) from the Mariana trench. Extremophiles 2006, 10, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Skropeta, D. Deep-sea natural products. Nat. Prod. Rep. 2008, 25, 1131–1166. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Schröder, H.C.; Feng, Q.; Draenert, F.; Müller, W.E.G. The deep-sea natural products, biogenic polyphosphate (bio-polyP) and biogenic silica (bio-silica), as biomimetic scaffolds for bone tissue engineering: Fabrication of a morphogenetically-active polymer. Mar. Drugs 2013, 11, 718–746. [Google Scholar] [CrossRef] [PubMed]
- Skropeta, D.; Wei, L. Recent advances in deep-sea natural products. Nat. Prod. Rep. 2014, 31, 999–1025. [Google Scholar] [CrossRef] [PubMed]
- Pettit, R.K. Culturability and secondary metabolite diversity of extreme microbes: Expanding contribution of deep sea and deep-sea vent microbes to natural product discovery. Mar. Biotechnol. 2011, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Lin, H.; Proksch, P.; Tang, X.; Shao, Z.; Lin, W. Microbacterins A and B, new peptaibols from the deep sea actinomycete Microbacterium sediminis sp. nov. YLB-01(T). Org. Lett. 2015, 17, 1220–1223. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, J.; Lin, X.; Zhao, B.; Wei, X.; Li, G.; Kaliaperumal, K.; Liao, S.; Yang, B.; Zhou, X.; et al. Three dimeric nitrophenyl trans-epoxyamides produced by the deep-sea-derived fungus Penicillium chrysogenum SCSIO41001. Org. Lett. 2016, 18, 3650–3653. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mei, X.-G.; Zhu, W.-M. New natural products from the marine-derived Streptomyces actinobacteria. Stud. Mar. Sin. 2016, 51, 86–124. [Google Scholar]
- Hughes, C.C.; MacMillan, J.B.; Gaudêncio, S.P.; Jensen, P.R.; Fenical, W. The ammosamides: Structures of cell cycle modulators from a marine-derived Streptomyces species. Angew. Chem. Int. Ed. 2009, 48, 725–727. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-G.; Song, N.-K.; Yoo, I.-D. Trienomycin G, a new inhibitor of nitric oxide production in microglia cells, from Streptomyces sp. 91614. J. Antibiot. 2002, 55, 204–207. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Shen, Y.; Bai, L. Biosynthesis of 3,5-AHBA-derived natural products. Nat. Prod. Rep. 2012, 29, 243–263. [Google Scholar] [CrossRef] [PubMed]
- Margalith, P.; Beretta, G. Rifomycin. XI. Taxonomic study on Streptomyces mediterranei nov. sp. Mycopathol. Mycol. Appl. 1960, 13, 321–330. [Google Scholar] [CrossRef]
- DeBoer, C.P.; Meulman, A.; Wnuk, R.J.; Peterson, D.H. Geldanamycin, a new antibiotic. J. Antibiot. 1970, 23, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.C.; Nam, S.-J.; Gulder, T.A.M.; Kauffman, A.A.; Jensen, P.R.; Fenical, W.; Moore, B.S. Structure and biosynthesis of the marine Streptomycete ansamycin ansalactam A and its distinctive branched chain polyketide extender unit. J. Am. Chem. Soc. 2011, 133, 1971–1977. [Google Scholar] [CrossRef] [PubMed]
- Wrona, I.E.; Agouridas, V.; Panek, J.S. Design and synthesis of ansamycin antibiotics. C. R. Chim. 2008, 11, 1483–1522. [Google Scholar] [CrossRef]
- Brandt, G.E.L.; Blagg, B.S.J. Monoenomycin: A simplified trienomycin A analogue that manifests anticancer activity. ACS Med. Chem. Lett. 2011, 2, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-N.; Zhang, W.-J.; Bi, S.-F.; Jiao, R.-H.; Tan, R.-X.; Ge, H.-M. New ansamycin analogues from the mutant strain of Streptomyces seoulensis. J. Antibiot. 2015, 68, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, D.J.; Krische, M.J. Total Synthesis of (+)-Trienomycins A and F via C–C bond-forming hydrogenation and transfer hydrogenation. J. Am. Chem. Soc. 2013, 135, 10986–10989. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.N.; Jiao, R.H.; Zhang, W.J.; Zhao, G.Y.; Dou, H.; Jiang, R.; Zhang, A.H.; Hou, Y.Y.; Bi, S.F.; Ge, H.M.; et al. New ansamycin derivatives generated by simultaneous mutasynthesis. Org. Lett. 2015, 17, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Wehrli, W.; Staehelin, M. Actions of the rifamycins. Bacteriol. Rev. 1971, 35, 290–309. [Google Scholar] [PubMed]
- Pacey, S.; Gore, M.; Chao, D.; Banerji, U.; Larkin, J.; Sarker, S.; Owen, K.; Asad, Y.; Raynaud, F.; Walton, M.; et al. A Phase II trial of 17-allylamino, 17-demethoxygeldanamycin (17-AAG, tanespimycin) in patients with metastatic melanoma. Investig. New Drugs 2012, 30, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, L.; Fan, J.; Sun, K.; Zhu, W. Cytotoxic compounds from the deep-sea sediment-derived Streptomyces malaysiensis OUCMDZ-2167. Chin. J. Org. Chem. 2017, 37, 658–666. [Google Scholar] [CrossRef]
- Wang, C.; Xu, Y.-J.; Huang, X.-L.; Hao, J.-J.; Zhu, W.-M. Pyrrole alkaloids from the deep-sea sediment-derived Streptomyces sp. OUCMDZ-4112. Chin. J. Mar. Drugs 2016, 35, 1–9. [Google Scholar]
- Wang, C.; Monger, A.; Wang, L.; Fu, P.; Piyachaturawat, P.; Chairoungdua, A.; Zhu, W. Precursor-directed generation of indolocarbazoles with topoisomerase IIα inhibitory activity. Mar. Drugs 2018, 16, 168. [Google Scholar] [CrossRef] [PubMed]
- Funayama, S.; Anraku, Y.; Mita, A.; Yang, Z.B.; Shibata, K.; Komiyama, K.; Umezawa, I.; Omura, S. Structure-activity relationship of a novel antitumor ansamycin antibiotic trienomycin A and related compounds. J. Antibiot. 1988, 41, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.B., III; Wood, J.L.; Wong, W.; Gould, A.E.; Rizzo, C.J. (+)-Trienomycins A, B, and C: Relative and absolute stereochemistry. J. Am. Chem. Soc. 1990, 112, 7425–7426. [Google Scholar] [CrossRef]
- Nomoto, H.; Katsumata, S.; Takahashi, K.; Funayama, S.; Komiyama, K.; Umezawa, I.; Omura, S. Structural studies on minor components of trienomycin group antibiotics trienomycins D and E. J. Antibiot. 1989, 42, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.B., III; Wood, J.L.; Gould, A.E.; Omura, S.; Komiyama, K. Isolation and structure determination of (+)-trienomycin-f. An endgame synthetic strategy for the trienomycin family of antitumor antibiotics. Tetrahedron Lett. 1991, 32, 1627–1630. [Google Scholar] [CrossRef]
- Marfey, P. Determination of D-amino acids. II. Use of a bifunctional reagent, 1,5-difluoro-2,4-dinitrobenzene. Carlsberg Res. Commun. 1984, 49, 591–596. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Xiong, T.; Chen, X.; Wei, H.; Xiao, H. Influence of PJ34 on the genotoxicity induced by melphalan in human multiple myeloma cells. Arch. Med. Sci. 2015, 11, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Kong, F.; Wang, Y.; Fu, P.; Zhu, W. Cladodionen, a cytotoxic hybrid polyketide from the marine-derived Cladosporium sp. OUCMDZ-1635. Mar. Drugs 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]



| No. | 1 | 2 | ||
|---|---|---|---|---|
| δC | δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) | |
| 1 | 167.7, C | 167.6, C | ||
| 2 | 43.3, CH2 | 2.63, dd (11.6, 3.8); 2.34, m | 43.3, CH2 | 2.68, dd (12.0, 4.5); 2.34, m |
| 3 | 79.8, CH | 3.98, m | 79.8, CH | 3.99, ddd (10.9, 8.4, 4.6) |
| 3-OCH3 | 55.7, CH3 | 3.20, s | 55.6, CH3 | 3.21, s |
| 4 | 131.9, CH | 5.55, m a | 131.5, CH | 5.53, dd (15.0, 8.3) |
| 5 | 133.0, CH | 6.06, m a | 132.1, CH | 6.10, m a |
| 6 | 129.8, CH | 6.06, m a | 129.1, CH | 6.09, m a |
| 7 | 133.2, CH | 6.12, m a | 133.7, CH | 6.08, m a |
| 8 | 133.1, CH | 6.07, m a | 133.2, CH | 6.05, m a |
| 9 | 129.5, CH | 5.54, m a | 131.4, CH | 5.72, ddd (14.7, 10.1, 4.6) |
| 10 | 32.3, CH2 | 2.42, m; 2.21, m | 35.5, CH2 | 2.44, m; 2.33, m |
| 11 | 74.1, CH | 4.66, brs | 72.3, CH | 5.62, d (4.6) |
| 12 | 38.7, CH | 1.74, m | 39.1, CH | 1.71, m |
| 13 | 67.1, CH | 4.47, brs | 69.4, CH | 4.72, brs |
| 14 | 139.5, C | 139.5, C | ||
| 15 | 123.5, CH | 5.10, brs | 125.6, CH | 5.19, m |
| 16 | 28.8, CH2 | 2.23, m; 1.80, m | 29.0, CH2 | 2.20, m; 2.18, m |
| 17 | 35.8, CH2 | 2.44, m; 2.34, m | 36.2, CH2 | 2.44, m; 2.33, m |
| 18 | 142.9, C | 142.7, C | ||
| 19 | 111.2, CH | 6.30, s | 111.0, CH | 6.30, s |
| 20 | 139.5, C | 139.5, C | ||
| 21 | 105.3, CH | 6.85, s | 105.2, CH | 6.82, s |
| 22 | 157.3, C | 157.3, C | ||
| 23 | 111.2, CH | 6.43, s | 111.1, CH | 6.47, s |
| 24 | 9.7, CH3 | 0.79, d (6.5) | 10.2, CH3 | 0.78, d (6.0) |
| 25 | 20.6, CH3 | 1.66, s | 20.3, CH3 | 1.56, s |
| 1′ | 171.9, C | 169.8, C | ||
| 2′ | 48.2, CH | 4.19, dq (6.2, 7.2) | 20.7, CH3 | 2.00, s |
| 3′ | 16.9, CH3 | 1.24, d (7.2) | ||
| 4′ | 169.6, C | |||
| 5′ | 22.2, CH3 | 1.81, s | ||
| 20-NH | 9.54, s | 9.52, s | ||
| 2′-NH | 8.34, d (6.2) | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Wang, C.; Wang, L.; Chairoungdua, A.; Piyachaturawat, P.; Fu, P.; Zhu, W. New Ansamycins from the Deep-Sea-Derived Bacterium Ochrobactrum sp. OUCMDZ-2164. Mar. Drugs 2018, 16, 282. https://doi.org/10.3390/md16080282
Fan Y, Wang C, Wang L, Chairoungdua A, Piyachaturawat P, Fu P, Zhu W. New Ansamycins from the Deep-Sea-Derived Bacterium Ochrobactrum sp. OUCMDZ-2164. Marine Drugs. 2018; 16(8):282. https://doi.org/10.3390/md16080282
Chicago/Turabian StyleFan, Yaqin, Cong Wang, Liping Wang, Arthit Chairoungdua, Pawinee Piyachaturawat, Peng Fu, and Weiming Zhu. 2018. "New Ansamycins from the Deep-Sea-Derived Bacterium Ochrobactrum sp. OUCMDZ-2164" Marine Drugs 16, no. 8: 282. https://doi.org/10.3390/md16080282
APA StyleFan, Y., Wang, C., Wang, L., Chairoungdua, A., Piyachaturawat, P., Fu, P., & Zhu, W. (2018). New Ansamycins from the Deep-Sea-Derived Bacterium Ochrobactrum sp. OUCMDZ-2164. Marine Drugs, 16(8), 282. https://doi.org/10.3390/md16080282