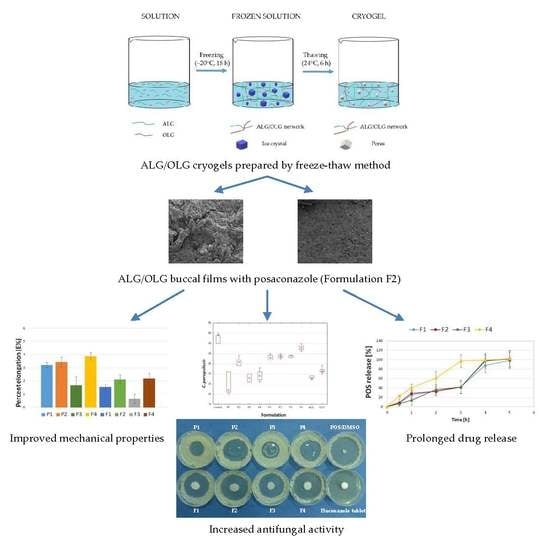
Alginate Oligosaccharides Affect Mechanical Properties and Antifungal Activity of Alginate Buccal Films with Posaconazole
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteristics of Buccal Films
2.2. Mechanical Properties
2.3. Swelling Study
2.4. Mucoadhesive Properties
2.5. In Vitro POS-Release
2.6. Differential Scanning Calorimetry (DSC)
2.7. Antifungal Activity
3. Materials and Methods
3.1. Materials
3.2. Films Preparations
3.2.1. Placebo Films Preparation Using Cryogels Obtained by the Freeze-Thaw Method
3.2.2. Solutions and Cryogels Viscosity Measurements
3.2.3. POS-Loaded Films Preparation Using Cryogels Obtained by the Freeze-Thaw Method
3.3. Films Evaluation
3.3.1. Scanning Electron Microscopy (SEM)
3.3.2. Surface pH
3.3.3. Film Thickness
3.3.4. Moisture Content
3.3.5. Disintegration Time
3.3.6. Mechanical Properties
3.3.7. Swelling Properties
3.3.8. Mucoadhesiveness
Ex Vivo Mucoadhesive Properties
Ex Vivo Residence Time
In Vivo Mucoadhesion Time
3.3.9. Drug Content Uniformity
3.3.10. High-Performance Liquid Chromatography (HPLC) Assay
3.3.11. In Vitro Drug Release
3.3.12. Drug Release Mechanisms
3.3.13. Differential Scanning Calorimetry (DSC)
3.3.14. Antifungal Activity
3.3.15. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scorzoni, L.; de Paula e Silva, A.C.A.; Marcos, C.M.; Assato, P.A.; de Melo, W.C.M.A.; de Oliveira, H.C.; Costa-Orlandi, C.B.; Mendes-Giannini, M.J.S.; Fusco-Almeida, A.M. Antifungal therapy: New advances in the understanding and treatment of mycosis. Front. Microbiol. 2017, 8, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolton, M.J.; Ray, J.E.; Chen, S.C.; Ng, K.; Pont, L.; McLachlan, A.J. Multicenter study of posaconazole therapeutic drug monitoring: Exposure-response relationship and factors affecting concentration. Antimicrob. Agents Chemother. 2012, 56, 5503–5510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, S.; Boddohi, S. New formulation and approach for mucoadhesive buccal film of rizatriptan benzoate. Prog. Biomater. 2017, 6, 175–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current use and future perspectives in pharmaceutical and biomedical applications. Int. J. Polym. Sci. 2016, 2016, 17. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, M.J.; Costa, R.R.; Mano, J.F. Marine origin polysaccharides in drug delivery systems. Mar. Drugs 2016, 14, 34. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.L.; Yang, M.; Fu, X.D.; Chen, M.; Su, Q.; Zhao, Y.H.; Mou, H.J. Evaluation of prebiotic potential of three marine algae oligosaccharides from enzymatic hydrolysis. Mar. Drugs 2019, 17, 173. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, M.F.; Powell, L.C.; Jack, A.A.; Powell, K.; Beck, K.; Florance, H.; Forton, J.; Rye, P.D.; Dessen, A.; Hill, K.E.; et al. A low-molecular-weight alginate oligosaccharide disrupts pseudomonal microcolony formation and enhances antibiotic effectiveness. Antimicrob. Agents Chemother. 2017, 61, e00762-17. [Google Scholar] [CrossRef] [Green Version]
- Tøndervik, A.; Sletta, H.; Klinkenberg, G.; Emanuel, C.; Powell, L.C.; Pritchard, M.F.; Khan, S.; Craine, K.M.; Onsøyen, E.; Rye, P.D.; et al. Alginate oligosaccharides inhibit fungal cell growth and potentiate the activity of antifungals against Candida and Aspergillus spp. PLoS ONE 2014, 19, e112518. [Google Scholar] [CrossRef] [Green Version]
- de Castro Spadari, C.; Lopes, L.B.; Ishida, K. Potential use of alginate-based carriers as antifungal delivery system. Front. Microbiol. 2017, 8, 97. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Shen, W.; Chen, Z.; Wu, T. Freeze-thaw induced gelation of alginates. Carbohydr. Polym. 2016, 148, 45–51. [Google Scholar] [CrossRef]
- Gurikov, P.; Smirnova, I. Non-conventional methods for gelation of alginate. Gels 2018, 4, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Dorado, R.; López-Iglesias, C.; García-González, C.A.; Auriemma, G.; Aquino, R.P.; Del Gaudio, P. Design of aerogels, cryogels and xerogels of alginate: Effect of molecular weight, gelation conditions and drying method on particles’ micromeritics. Molecules 2019, 24, 1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Council of Europe. The European Pharmacopeia, 9th ed.; Council of Europe: Strasburg, France, 2016; Volume 1, p. 302. [Google Scholar]
- Baliga, S.; Muglikar, S.; Kale, R. Salivary pH: A diagnostic biomarker. J. Indian Soc. Periodontol. 2013, 17, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef] [Green Version]
- Bahri-Najafi, R.; Tavakoli, N.; Senemar, M.; Peikanpour, M. Preparation and pharmaceutical evaluation of glibenclamide slow release mucoadhesive buccal film. Res. Pharm. Sci. 2014, 9, 213–223. [Google Scholar]
- Costa, M.J.; Marques, A.M.; Pastrana, L.M.; Teixeira, J.A.; Sillankorva, S.M.; Cerqueira, M.A. Physicochemical properties of alginate-based films: Effect of ionic cross-linking and mannuronic and guluronic acid ratio. Food Hydrocoll. 2018, 81, 442–448. [Google Scholar] [CrossRef] [Green Version]
- Bîrsan, M.; Apostu, M.; Todoran, N.; Antonoaea, P.; Rusu, A.; Ciurba, A. Development of dermal films containing miconazole nitrate. Molecules 2018, 23, 1640. [Google Scholar] [CrossRef] [Green Version]
- Ashikin, W.H.N.S.; Wong, T.W.; Law, C.L. Plasticity of hot air-dried mannuronate- and guluronate-rich alginate films. Carbohydr. Polym. 2010, 81, 104–113. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Magalhães, U.S.; Oliveira, S.A.; Ribeiro, H.L.; Brito, E.S.; De Moura, M.R. Tensile and water vapour properties of calcium-crosslinked alginate-cashew tree gum films. J. Food Sci. Technol. 2012, 47, 710–715. [Google Scholar] [CrossRef]
- Liang, H.; Li, S.; Lu, Y.; Yang, T. Reliability study on FRP composites exposed to wet-dry cycles. Appl. Sci. 2018, 8, 892. [Google Scholar] [CrossRef] [Green Version]
- Buanz, A.B.M.; Belaunde, C.C.; Soutari, N.; Tuleu, C.; Gul, M.O.; Gaisford, S. Ink-jet printing versus solvent casting to prepare oral films: Effect on mechanical properties and physical stability. Int. J. Pharm. 2015, 2, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Aggarwal, S. Mucoadhesive polymeric platform for drug delivery; a comprehensive review. Curr. Drug Deliv. 2015, 12, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.S.; Auffret, A.D.; Matthews, K.H.; Humphrey, M.J.; Stevens, H.N.; Eccleston, G.M. Characterisation of lyophilised wafers and solvent evaporated films as potential drug delivery systems to mucosal surfaces. Int. J. Pharm. 2010, 389, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Sarheed, O.; Rasool, B.K.; Abu-Gharbieh, E.; Aziz, U.S. An investigation and characterization on alginate hydrogel dressing loaded with metronidazole prepared by combined inotropic gelation and freeze-thawing cycles for controlled release. AAPS PharmSciTech 2015, 16, 601–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fennel, E.; Huyghe, J.M. Chemically responsive hydrogel deformation mechanics: A review. Molecules 2019, 24, 3521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaikh, R.; Singh, T.R.R.; Garland, M.J.; Woolfson, D.A.; Donnelly, R.F. Mucoadhesive drug delivery systems. J. Pharm. Bioallied. Sci. 2011, 3, 89–100. [Google Scholar] [CrossRef]
- Amemiya, T.; Nakamura, T.; Yamamoto, T.; Kinoshita, S.; Kanamura, N. Autologous transplantation of oral mucosal epithelial cell sheets cultured on an amniotic membrane substrate for intraoral mucosal defects. PLoS ONE 2015, 10, e0125391. [Google Scholar] [CrossRef] [Green Version]
- Menchicchi, B.; Fuenzalida, J.P.; Hensel, A.; Swamy, M.J.; David, L.; Rochas, C.; Goycoolea, F.M. Biophysical analysis of the molecular interactions between polysaccharides and mucin. Biomacromolecules 2015, 16, 924–935. [Google Scholar] [CrossRef]
- Çelik, B. Risperidone mucoadhesive buccal tablets: Formulation design, optimization and evaluation. Drug Des. Dev. Ther. 2017, 11, 3355–3365. [Google Scholar] [CrossRef] [Green Version]
- Nehme, H.; Saulnier, P.; Ramadan, A.A.; Cassisa, V.; Guillet, C.; Eveillard, M.; Umerska, A. Antibacterial activity of antipsychotic agents, their association with lipid nanocapsules and its impact on the properties of the nanocarriers and on antibacterial activity. PLoS ONE 2018, 13, e0189950. [Google Scholar] [CrossRef]
- Pishnamazi, M.; Hafizi, H.; Shirazian, S.; Culebras, M.; Walker, G.M.; Collins, M.N. Design of controlled release system for paracetamol based on modified lignin. Polymers 2019, 11, 1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szekalska, M.; Sosnowska, K.; Zakrzeska, A.; Kasacka, I.; Lewandowska, A.; Winnicka, K. The influence of chitosan cross-linking on the properties of alginate microparticles with metformin hydrochloride—In vitro and in vivo evaluation. Molecules 2017, 22, 182. [Google Scholar] [CrossRef] [PubMed]
- Skulason, S.; Asgeirsdottir, M.S.; Magnusson, J.P.; Kristmundsdottir, T. Evaluation of polymeric films for buccal drug delivery. Pharmazie 2009, 64, 197–201. [Google Scholar] [PubMed]
- Rana, P.; Murthy, R.S. Formulation and evaluation of mucoadhesive buccal films impregnated with carvedilol nanosuspension: A potential approach for delivery of drugs having high first-pass metabolism. Drug Deliv. 2013, 20, 224–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, P.; Sousa Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Gill, P.; Moghadam, T.T.; Ranjbar, B.; Chiu, M.H.; Jrenner, E.J. Differential scanning calorimetry: An invaluable tool for a detailed thermodynamic characterization of macromolecules and their interactions. J. Pharm. Bioallied. Sci. 2011, 3, 39–59. [Google Scholar] [CrossRef]
- Pathak, T.S.; Kim, J.S.; Lee, S.J.; Baek, D.J.; Paeng, K.J. Preparation of alginic acid and metal alginate from algae and their comparative study. J. Polym. Environ. 2008, 16, 198–204. [Google Scholar] [CrossRef]
- Yang, M.; Dong, Z.; Zhang, Y.; Zhang, F.; Wang, Y.; Zhao, Z. Preparation and evaluation of posaconazole-loaded enteric microparticles in rats. Drug Dev. Ind. Pharm. 2017, 43, 618–627. [Google Scholar] [CrossRef]
- DiNunzio, J.C.; Miller, D.A.; Yang, W.; McGinity, J.W.; Williams, R.O. Amorphous compositions using concentration enhancing polymers for improved bioavailability of itraconazole. Mol. Pharm. 2008, 5, 968–980. [Google Scholar] [CrossRef]
- Gupta, S.; Goswami, S.; Sinha, A. Acombined effect of freeze-thaw cycles and polymer concentration on the structure and mechanical properties of transparent PVA gels. Biomed. Mater. 2012, 7, 015006. [Google Scholar] [CrossRef]
- Pritchard., M.F.; Powell, L.C.; Khan, S.; Griffiths, P.C.; Mansour, O.T.; Schweins, R.; Beck, K.; Buurma, N.J.; Dempsey, C.E.; Wright, C.J.; et al. The antimicrobial effects of the alginate oligomer OligoG CF-5/20 are independent of direct bacterial cell membrane disruption. Sci. Rep. 2017, 7, 44731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rex, J.H. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; CLSI DocumentM27-A3; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Ahmadi, A.; Moghadamtousi, S.Z.; Abubakar, S.; Zandi, K. Antiviral potential of algae polysaccharides isolated from marine sources: A review. BioMed Res. Int. 2015, 2015, 825203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, J.; Ma, D.; Qin, W.; Liu, Y. Physical and antibacterial properties of sodium alginate-sodium carboxymethylcellulose films containing Lactococcus lactis. Molecules 2018, 23, 2645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, M.R.C.; Löbenberg, R.; Almukainzi, M. Simulated biological fluids with possible application in dissolution testing. Dissolution Technol. 2011, 3, 15–28. [Google Scholar] [CrossRef]
- Wroblewska, M.; Winnicka, K. The effect of cationic polyamidoamine dendrimers on physicochemical characteristics of hydrogels with erythromycin. Int. J. Mol. Sci. 2015, 16, 20277–20289. [Google Scholar] [CrossRef] [Green Version]
- Preis, M.; Pein, M.; Breitkreutz, J. Development of a taste-masked orodispersible film containing dimenhydrinate. Pharmaceutics 2012, 4, 551–562. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Vijaya, C. Formulation development of mouth dissolving film of etoricoxib for pain management. Adv. Pharm. 2015, 2015, 702963. [Google Scholar] [CrossRef]
- Kaur, K.; Naeem, M.; Ali, A.; Rehman, N.U.; Nawaz, Z.; Akram, M.R.; Khan, J.A. Assessment of guar and xanthan gum based floating drug delivery system containing mefenamic acid. Acta Pol. Pharm. 2016, 5, 1287–1297. [Google Scholar]
- Nakamura, F.; Ohta, R.; Machida, Y.; Nagai, T. In vitro and in vivo nasal mucoadhesion of some water-soluble polymers. Int. J. Pharm. 1996, 134, 173–181. [Google Scholar] [CrossRef]
- Tang, P.H. Determination of posaconazole in plasma/serum by high-performance liquid chromatography with fluorescence detection. Separations 2017, 4, 16. [Google Scholar] [CrossRef]
- Cansizoglu, M.F.; Tamer, Y.T.; Farid, M.; Koh, A.Y.; Toprak, E. Rapid ultrasensitive detection platform for antimicrobial susceptibility testing. PLoS Biol. 2019, 17, e3000291. [Google Scholar] [CrossRef] [PubMed]














| Formulation | Viscosity (mPa∙s)* |
|---|---|
| Solutions | |
| P1sol | 3803.3 ± 57.7 |
| P2sol | 4028.4 ± 112.9 |
| P3sol | 48651.0 ± 114.3 |
| P4sol | 4291.0 ± 125.0 |
| Cryogels | |
| P1gel | 5473.8 ± 290.6 |
| P2gel | 6685.0 ± 290.2 |
| P3gel | 96610.0 ± 2737.1 |
| P4gel | 7679.0 ± 299.6 |
| Formulation | ALG (g) | OLG (g) | GLY (g) | POS (g) | Purified Water (up to; g) |
|---|---|---|---|---|---|
| P1 | 2 | - | 0.6 | - | 100 |
| P2 | 1 | 1 | 0.6 | - | 100 |
| P3 | 2 | 2 | 0.6 | - | 100 |
| P4 | - | 2 | 0.6 | - | 100 |
| F1 | 2 | - | 0.6 | 3.27 | 100 |
| F2 | 1 | 1 | 0.6 | 3.27 | 100 |
| F3 | 2 | 2 | 0.6 | 3.27 | 100 |
| F4 | - | 2 | 0.6 | 3.27 | 100 |
| Formulation | Thickness (μm) | Surface pH | Disintegration Time (min) | Moisture Content (%) | Drug Content (mg) | |
|---|---|---|---|---|---|---|
| Conventional Apparatus | on Petri Dish | |||||
| P1 | 52.0 ± 5.4 | 7.1 ± 0.1 | 3.4 ± 0.3 | >240 | 8.8 ± 3.9 | - |
| P2 | 54.7 ± 8.2 | 7.0 ± 0.1 | 3.2 ± 0.1 | 120 ± 5 | 9.1 ± 1.9 | - |
| P3 | 76.7 ± 9.4 | 6.9 ± 0.1 | 6.5 ± 0.1 | 120 ± 5 | 9.4 ± 0.5 | - |
| P4 | 42.6 ± 5.7 | 6.9 ± 0.1 | 2.2 ± 0.1 | 120 ± 5 | 7.3 ± 1.6 | - |
| F1 | 213.8 ± 6.9 | 7.3 ± 0.3 | 16.1 ± 0.1 | >240 | 7.2 ± 1.2 | 95.4 ± 0.1 |
| F2 | 260.3 ± 8.6 | 7.2 ± 0.1 | 16.4 ± 0.1 | >240 | 5.3 ± 1.5 | 101.8 ± 9.2 |
| F3 | 287.0 ± 4.4 | 6.7 ± 0.1 | 19.2 ± 0.2 | >240 | 9.7 ± 0.2 | 103.8 ± 6.2 |
| F4 | 220.0 ± 8.1 | 6.7 ± 0.2 | 15.7 ± 0.5 | >240 | 9.6 ± 0.4 | 103.1 ± 6.5 |
| Formulation | Ex Vivo Residence Time (min) | In Vivo Residence Time (min) |
|---|---|---|
| P1 | 133.33 ± 10.41 | 129.67 ± 10.02 |
| P2 | 39.00 ± 5.29 | 50.33 ± 10.22 |
| P3 | 111.33 ± 6.03 | 153.50 ± 1.80 |
| P4 | 21.00 ± 3.61 | 54.17 ± 3.25 |
| F1 | 205.00 ± 10.00 | –* |
| F2 | 117.67 ± 7.51 | –* |
| F3 | 180.67 ± 14.98 | –* |
| F4 | 111.33 ± 4.04 | –* |
| Formu-Lation | Zero Order Kinetics | First Order Kinetics | Higuchi Model | Korsmeyer–Peppas Model | Hixson–Crowell Model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | K | R2 | K | R2 | K | R2 | K | n | R2 | K | |
| F1 | 0.94 | 19.98 | 0.80 | 0.81 | 0.89 | 57.81 | 0.93 | 0.48 | 0.65 | 0.87 | 5.18 |
| F2 | 0.89 | 20.88 | 0.64 | 0.94 | 0.85 | 60.29 | 0.90 | 0.48 | 0.63 | 0.82 | 5.76 |
| F3 | 0.94 | 23.68 | 0.67 | 0.89 | 0.89 | 68.39 | 0.97 | 0.49 | 0.80 | 0.80 | 6.11 |
| F4 | 0.94 | 22.39 | 0.84 | 1.27 | 0.97 | 67.29 | 0.95 | 0.50 | 0.47 | 0.94 | 1.60 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szekalska, M.; Wróblewska, M.; Trofimiuk, M.; Basa, A.; Winnicka, K. Alginate Oligosaccharides Affect Mechanical Properties and Antifungal Activity of Alginate Buccal Films with Posaconazole. Mar. Drugs 2019, 17, 692. https://doi.org/10.3390/md17120692
Szekalska M, Wróblewska M, Trofimiuk M, Basa A, Winnicka K. Alginate Oligosaccharides Affect Mechanical Properties and Antifungal Activity of Alginate Buccal Films with Posaconazole. Marine Drugs. 2019; 17(12):692. https://doi.org/10.3390/md17120692
Chicago/Turabian StyleSzekalska, Marta, Magdalena Wróblewska, Monika Trofimiuk, Anna Basa, and Katarzyna Winnicka. 2019. "Alginate Oligosaccharides Affect Mechanical Properties and Antifungal Activity of Alginate Buccal Films with Posaconazole" Marine Drugs 17, no. 12: 692. https://doi.org/10.3390/md17120692
APA StyleSzekalska, M., Wróblewska, M., Trofimiuk, M., Basa, A., & Winnicka, K. (2019). Alginate Oligosaccharides Affect Mechanical Properties and Antifungal Activity of Alginate Buccal Films with Posaconazole. Marine Drugs, 17(12), 692. https://doi.org/10.3390/md17120692