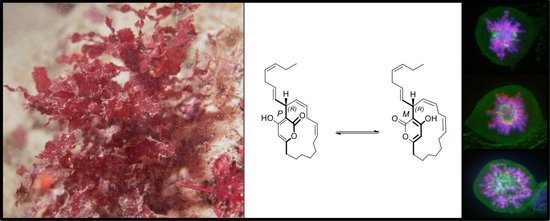
Neurymenolide A, a Novel Mitotic Spindle Poison from the New Caledonian Rhodophyta Phacelocarpus neurymenioides
Abstract
:1. Introduction
2. Results
2.1. Neurymenolide A Has Mild to Moderate Impact on the Growth of Cancerous Human Cell Lines
2.2. Neurymenolide A Induces Necrosis and Apoptosis in U-2 OS Human Osteosarcoma Cells
2.3. Neurymenolide A Induces Mitotic Spindle Destabilization at Prometaphase in U-2 OS Human Osteosarcoma Cells
2.4. Neurymenolide A Induces a Delay of Microtubule Repolymerization in U-2 OS Human Osteosarcoma Cells
2.5. Neurymenolide A Has R Absolute Configuration at Position C-17
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Biological Material
4.3. Extraction and Isolation of Neurymenolide A
Neurymenolide A
4.4. Cell Lines and Cell Culture Conditions
4.5. Cell Treatments
4.6. MTS Reduction Assay
4.7. Flow Cytometry Analysis
4.8. Immunofluorescence Staining
4.9. Time-Lapse Movies
4.10. Microtubule Regrowth Assay
4.11. Circular Dichroism Data
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Abrams, J.M.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; Dawson, T.M.; Dawson, V.L.; El-Deiry, W.S.; Fulda, S.; et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012, 19, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Mc Gee, M.M. Targeting the mitotic catastrophe signaling pathway in cancer. Mediators Inflamm. 2015, 2015, 146282. [Google Scholar] [CrossRef] [PubMed]
- Sharp, D.J.; Rogers, G.C.; Scholey, J.M. Microtubule motors in mitosis. Nature 2000, 407, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Checchi, P.M.; Nettles, J.H.; Zhou, J.; Snyder, J.P.; Joshi, H.C. Microtubule-interacting drugs for cancer treatment. Trends Pharmacol. Sci. 2003, 24, 361–365. [Google Scholar] [CrossRef]
- Gascoigne, K.E.; Taylor, S.S. How do anti-mitotic drugs kill cancer cells? J. Cell Sci. 2009, 122, 2579–2585. [Google Scholar] [CrossRef] [Green Version]
- Marzo, I.; Naval, J. Antimitotic drugs in cancer chemotherapy: Promises and pitfalls. Biochem. Pharmacol. 2013, 86, 703–710. [Google Scholar] [CrossRef]
- Marine Pharmaceuticals: The Clinical Pipeline. Available online: http://marinepharmacology.midwestern.edu/clinPipeline.htm (accessed on 8 January 2018).
- Swami, U.; Chaudhary, I.; Ghalib, M.H.; Goel, S. Eribulin—A review of preclinical and clinical studies. Crit. Rev. Oncol. Hematol. 2012, 81, 163–184. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.J.; Zheng, W.; Seletsky, B.M. From micrograms to grams: Scale-up synthesis of eribulin mesylate. Nat. Prod. Rep. 2013, 30, 1158–1164. [Google Scholar] [CrossRef]
- Pettit, G.R.; Kamano, Y.; Herald, C.L.; Tuinman, A.A.; Boettner, F.E.; Kizu, H.; Schmidt, J.M.; Baczynskyj, L.; Tomer, K.B.; Bontems, R.J. The isolation and structure of a remarkable marine animal antineoplastic constituent: Dolastatin 10. J. Am. Chem. Soc. 1987, 109, 6883–6885. [Google Scholar] [CrossRef]
- Luesch, H.; Moore, R.E.; Paul, V.J.; Mooberry, S.L.; Corbett, T.H. Isolation of dolastatin 10 from the marine cyanobacterium Symploca species VP642 and total stereochemistry and biological evaluation of its analogue symplostatin 1. J. Nat. Prod. 2001, 64, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Stout, E.P.; Hasemeyer, A.P.; Lane, A.L.; Davenport, T.M.; Engel, S.; Hay, M.E.; Fairchild, C.R.; Prudhomme, J.; Le Roch, K.; Aalbersberg, W.; et al. Antibacterial neurymenolides from the Fijian red alga Neurymenia fraxinifolia. Org. Lett. 2009, 11, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Andras, T.D.; Alexander, T.S.; Gahlena, A.; Parry, R.M.; Fernandez, F.M.; Kubanek, J.; Wang, M.D.; Hay, M.E. Seaweed allelopathy against coral: Surface distribution of a seaweed secondary metabolite by imaging mass spectrometry. J. Chem. Ecol. 2012, 38, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Chaładaj, W.; Corbet, M.; Fürstner, A. Total Synthesis of Neurymenolide A Based on a Gold-Catalyzed Synthesis of 4-Hydroxy-2-pyrones. Angew. Chem. Int. Ed. 2012, 124, 7035–7039. [Google Scholar] [CrossRef]
- Fürstner, A. From Understanding to Prediction: Gold-and Platinum-Based π-Acid Catalysis for Target Oriented Synthesis. Acc. Chem. Res. 2013, 47, 925–938. [Google Scholar]
- Motuhi, S.-E.; Mehiri, M.; Payri, C.E.; La Barre, S.; Bach, S. Marine Natural Products from New Caledonia—A Review. Mar. Drugs 2016, 14, 58. [Google Scholar] [CrossRef]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009, 29, 2993–2996. [Google Scholar]
- Sirri, V.; Roussel, P.; Hernandez-Verdun, D. In vivo release of mitotic silencing of ribosomal gene transcription does not give rise to precursor ribosomal RNA processing. J Cell Biol. 2000, 148, 259–270. [Google Scholar] [CrossRef]
- Nugroho, A.E.; Morita, H. Circular dichroism calculation for natural products. J. Nat. Med. 2014, 68, 1–10. [Google Scholar] [CrossRef]
- Gardner, M.K.; Zanic, M.; Howard, J. Microtubule catastrophe and rescue. Curr. Opin. Cell Biol. 2013, 25, 14–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doménech, E.; Malumbres, M. Mitosis-targeting therapies: A troubleshooting guide. Curr. Opin. Pharmacol. 2013, 13, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Winey, M. The MPS1 family of protein kinases. Annu. Rev. Biochem. 2012, 81, 561–585. [Google Scholar] [CrossRef] [PubMed]
- Malumbres, M. Physiological relevance of cell cycle kinases. Physiol. Rev. 2011, 91, 973–1007. [Google Scholar] [CrossRef]
- Kelly, A.E.; Funabiki, H. Correcting aberrant kinetochore microtubule attachments: An Aurora B-centric view. Curr. Opin. Cell Biol. 2009, 21, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Glunz, P.W. Recent encounters with atropisomerism in drug discovery. Bioorg. Med. Chem. Lett. 2018, 28, 53–60. [Google Scholar] [CrossRef]
- Smith, D.E.; Marquez, I.; Lokensgard, M.E.; Rheingold, A.L.; Hecht, D.A.; Gustafson, J.L. Exploiting atropisomerism to increase the target selectivity of kinase inhibitors. Angew. Chem. Int. Ed. 2015, 54, 11754–11759. [Google Scholar] [CrossRef]
- Ma, H.T.; Erdal, S.; Huang, S.; Poon, R.Y. Synergism between inhibitors of Aurora A and KIF11 overcomes KIF15-dependent drug resistance. Mol. Oncol. 2014, 8, 1404–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liston, D.R.; Davis, M. Clinically relevant concentrations of anticancer drugs: A guide for nonclinical studies. Clin Cancer Res. 2017, 23, 3489–3498. [Google Scholar] [CrossRef] [PubMed]
- Fant, X.; Gnadt, N.; Haren, L.; Merdes, A. Stability of the small γ-tubulin complex requires HCA66, a protein of the centrosome and the nucleolus. J. Cell Sci. 2009, 122, 1134–1144. [Google Scholar] [CrossRef] [PubMed]











| Cancer Type | Cell Line | IC50 (µM) | SI ** |
|---|---|---|---|
| Solid malignancies | U-2 OS | 102.8 ± 1 (46.5) * | 2.4 |
| MCF-7 | 107.4 | 2.3 | |
| IMR-32 | 181.9 ± 1 | 1.3 | |
| Hep G2 | 192.1 | 1.3 | |
| PANC-1 | 270.6 ± 2 | 0.9 | |
| HT-29 | 362.1 | 0.7 | |
| AsPC-1 | 446.5 ± 1 | 0.6 | |
| Hematological malignancies | A3 | 73.3 (27.9) * | 3.3 |
| THP-1 | 99.5 | 2.5 | |
| K-562/ADR | 243.6 ± 1 | 1.0 | |
| K-562 | 398.6 ± 1 | 0.6 | |
| Non-malignant | HEK-293 | 243.6 | - |
| Natural Products | Synthetic | |||
|---|---|---|---|---|
| N° | δc (ppm) a | δc (ppm) b | Δδc | δc (ppm) c |
| 1 | 165.1 | 165.1 | 0 | 165.1 |
| 2 | 103.8 | 103.9 | 0.1 | 103.8 |
| 3 | 164.5 | 164.7 | 0.2 | 164.7 |
| 4 | 101.3 | 101.4 | 0.1 | 101.3 |
| 5 | 165.1 | 165.1 | 0 | 165.1 |
| 6 | 33.5 | 33.5 | 0 | 33.5 |
| 7 | 25.6 | 25.6 | 0 | 25.6 |
| 8 | 26.9 | 26.9 | 0 | 26.9 |
| 9 | 27.7 | 27.7 | 0 | 27.7 |
| 10 | 27.1 | 27.1 | 0 | 27.1 |
| 11 | 26.6 | 26.6 | 0 | 26.6 |
| 12 | 131.0 | 131.0 | 0 | 131.0 |
| 13 | 126.6 | 126.6 | 0 | 126.6 |
| 14 | 27.0 | 27.0 | 0 | 27.0 |
| 15 | 135.6 | 135.2 | −0.4 | 135.5 |
| 16 | 126.8 | 127.0 | 0.2 | 126.9 |
| 17 | 36.5 | 36.5 | 0 | 36.5 |
| 18 | 129.4 | 129.4 | 0 | 129.4 |
| 19 | 129.9 | 129.9 | 0 | 129.9 |
| 20 | 30.0 | 30.0 | 0 | 30.0 |
| 21 | 125.9 | 125.9 | 0 | 125.9 |
| 22 | 133.0 | 132.9 | −0.1 | 133.0 |
| 23 | 20.5 | 20.5 | 0 | 20.5 |
| 24 | 14.2 | 14.2 | 0 | 14.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motuhi, S.-E.; Feizbakhsh, O.; Foll-Josselin, B.; Baratte, B.; Delehouzé, C.; Cousseau, A.; Fant, X.; Bulinski, J.C.; Payri, C.E.; Ruchaud, S.; et al. Neurymenolide A, a Novel Mitotic Spindle Poison from the New Caledonian Rhodophyta Phacelocarpus neurymenioides. Mar. Drugs 2019, 17, 93. https://doi.org/10.3390/md17020093
Motuhi S-E, Feizbakhsh O, Foll-Josselin B, Baratte B, Delehouzé C, Cousseau A, Fant X, Bulinski JC, Payri CE, Ruchaud S, et al. Neurymenolide A, a Novel Mitotic Spindle Poison from the New Caledonian Rhodophyta Phacelocarpus neurymenioides. Marine Drugs. 2019; 17(2):93. https://doi.org/10.3390/md17020093
Chicago/Turabian StyleMotuhi, Sofia-Eléna, Omid Feizbakhsh, Béatrice Foll-Josselin, Blandine Baratte, Claire Delehouzé, Arnaud Cousseau, Xavier Fant, Jeannette Chloë Bulinski, Claude Elisabeth Payri, Sandrine Ruchaud, and et al. 2019. "Neurymenolide A, a Novel Mitotic Spindle Poison from the New Caledonian Rhodophyta Phacelocarpus neurymenioides" Marine Drugs 17, no. 2: 93. https://doi.org/10.3390/md17020093
APA StyleMotuhi, S.-E., Feizbakhsh, O., Foll-Josselin, B., Baratte, B., Delehouzé, C., Cousseau, A., Fant, X., Bulinski, J. C., Payri, C. E., Ruchaud, S., Mehiri, M., & Bach, S. (2019). Neurymenolide A, a Novel Mitotic Spindle Poison from the New Caledonian Rhodophyta Phacelocarpus neurymenioides. Marine Drugs, 17(2), 93. https://doi.org/10.3390/md17020093