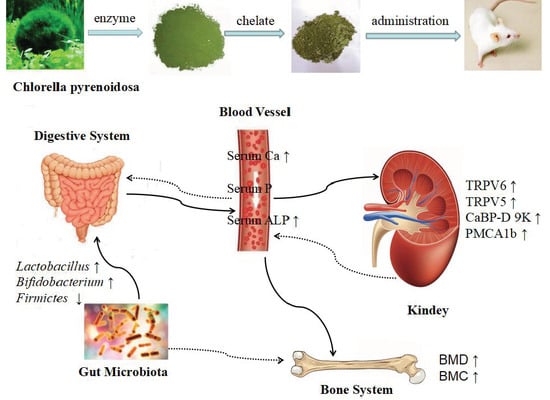
Effect of Chlorella Pyrenoidosa Protein Hydrolysate-Calcium Chelate on Calcium Absorption Metabolism and Gut Microbiota Composition in Low-Calcium Diet-Fed Rats
Abstract
:1. Introduction
2. Results
2.1. Characterization of Potent Major Compounds of CPPH and Structural Characterization of CPPH-Ca
2.2. Body Weight and Biochemical Parameters in Serum
2.3. Bone Biomechanical Parameters and Histomorphometry
2.4. Calcium Balance
2.5. Gene Expression of Corresponding Receptors in the Kidney of the Rats
2.6. CPPH-Ca Modulates Caecal Microbiota Composition of Calcium-Deficient Rats
2.7. Correlations of Biochemical Data and Key Phylotypes of Caecal Microbiota
3. Discussion
4. Materials and Methods
4.1. Preparation of CPPH, CPPH-Ca and HPLC-MS/MS Analysis
4.2. Structural Characterization of Peptide-Calcium Chelate by Ultraviolet Spectroscopy and FTIR Analysis
4.3. Animals
4.4. Sample Collection
4.5. Serum Calcium, Phosphorus Content and ALP Activity
4.6. Bone Index Parameter and Histomorphometry Analysis
4.7. Calcium Balance Study
4.8. RT-qPCR Analysis
4.9. Extraction of Caecal Genomic DNA for High Throughput Sequencing
4.10. Bioinformatics Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nicklas, T.A. Calcium intake trends and health consequences from childhood through adulthood. J. Am. Coll. Nutr. 2003, 22, 340–356. [Google Scholar] [CrossRef] [PubMed]
- Zemel, M.B.; Miller, S.L. Dietary calcium and dairy modulation of adiposity and obesity risk. Nutr. Rev. 2004, 62, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D. Calcium intake, calcium bioavailability and bone health. Br. J. Nutr. 2002, 87, 169–177. [Google Scholar] [CrossRef]
- Chen, D.; Mu, X.M.; Huang, H.; Nie, R.Y.; Liu, Z.Y.; Zeng, M.Y. Isolation of a calcium-binding peptide from tilapia scale protein hydrolysate and its calcium bioavailability in rats. J. Func. Foods 2014, 6, 575–584. [Google Scholar] [CrossRef]
- Benzvi, L.; Gershon, A.; Lavi, I.; Wollstein, R. Secondary prevention of osteoporosis following fragility fractures of the distal radius in a large health maintenance organization. Arch. Osteoporos. 2016, 11, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Harvey, N.C.; Curtis, E.M.; Velde, R. On epidemiology of fractures and variation with age and ethnicity. Bone 2016, 93, 230–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aeberli, D.; Eser, P.; Bonel, H.; Widmer, J.; Caliezi, G.; Varisco, P.A.; Möller, B.; Villiger, P.M. Reduced trabecular bone mineral density and cortical thickness accompanied by increased outer bone circumference in metacarpal bone of rheumatoid arthritis patients: A cross-sectional study. Arthritis Res. Ther. 2010, 12, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, X.; Ai, T.; Cheng, R.X.; Guo, H.Y.; Teng, G.X.; Mao, X.Y. Preparation and characterization of b-lactoglobulin hydrolysate-iron complexes. J. Dairy Sci. 2012, 95, 4230–4236. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.A.; Kenny, P.; Poindexter, J.; Pak, C.Y. Superior calcium absorption from calcium citrate than calcium carbonate using external forearm counting. J. Am. Coll. Nutr. 1990, 9, 583–587. [Google Scholar] [CrossRef]
- Cosentino, S.; Donida, B.M.; Marasco, E.; Favero, E.D.; Cantu, L.; Lombardi, G.; Colmbini, A.; Lametti, S.; Valaperta, S.; Fiorilli, A.; et al. Calcium ions enclosed in casein phosphopeptide aggregates are directly involved in the mineral uptake by differentiated HT-29 cells. Int. Dairy J. 2010, 20, 770–776. [Google Scholar] [CrossRef]
- Holt, C.; Timmins, P.A.; Errington, N.; Leaver, J. A core-shell model of calcium phosphate nanoclusters stabilized by β-casein phosphopeptides, derived from sedimentation equilibrium and small-angle X-ray and neutron-scattering measurements. Eur. J. Biochem. 1998, 252, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Noguchi, T.; Naito, H. An enhanced intestinal absorption of calcium in the rat directly attributed to dietary casein. Agric. Biol. Chem. 1979, 43, 2009–2011. [Google Scholar]
- Zhao, L.N.; Cai, X.X.; Huang, S.L.; Wang, S.Y.; Huang, Y.F.; Hong, J.; Rao, P.F. Isolation and identification of a whey protein-sourced calcium-binding tripeptide Tyr-Asp-Thr. Inter. Dairy J. 2015, 40, 16–23. [Google Scholar] [CrossRef]
- Bao, X.L.; Lv, Y.; Yang, B.C.; Ren, C.G.; Guo, S.T. A study of the soluble complexes formed during calcium binding bysoybean protein hydrolysates. J. Food Sci. 2008, 73, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.K.; Kim, S.K. Calcium-binding peptide derived from pepsinolytic hydrolysates of hoki (Johnius belengerii) frame. Eur. Food Res. Technol. 2007, 224, 763–767. [Google Scholar] [CrossRef]
- Huang, G.G.; Ren, L.; Jiang, J.X. Purification of a histidine-containing peptide with calcium binding activity from shrimp processing byproducts hydrolysate. Eur. Food Res. Technol. 2011, 232, 281–287. [Google Scholar] [CrossRef]
- Pan, D.D.; Lu, H.Q.; Zeng, X.Q. A newly isolated Ca binding peptide from whey protein. Int. J. Food Prop. 2013, 16, 1127–1134. [Google Scholar] [CrossRef]
- Han, Y.; He, H.; Zhao, N.N.; Li, J.T.; Wang, Z.Z.; Nie, Z.K. Effect of egg white peptide binding with calcium on promotion of calcium absorption in vivo. Food Sci. 2012, 33, 262–265. [Google Scholar]
- Ferraretto, A.; Signorile, A.; Gravaghi, C.; Tettamanti, G. Casein phosphopeptides influence calcium uptake by cultured human intestinal HT-29 tumor cells. J. Nutr. 2001, 131, 1655–1661. [Google Scholar] [CrossRef]
- Zhen, D.; Liu, L.; Guan, C.; Zhao, N.; Tang, X. High prevalence of vitamin D defciency among middle-aged and elderly individuals in northwestern China: Its relationship to osteoporosis and lifestyle factors. Bone 2015, 71, 1–6. [Google Scholar] [CrossRef]
- Guzman, S.; Gato, A.; Calleja, J.M. Antiinflammatory, analgesic and free radical scavenging activities of the marine microalgae Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother. Res. 2001, 15, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Nakamura, K.; Inoue, H. Therapeutic potentials of unicellular green alga Chlorella in advanced glycation end product (AGE)-related disorders. Med. Hypotheses 2005, 65, 953–955. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Kwon, H.J.; Kim, M.K. Hypoglycemic effect of Chlorella vulgaris intake in type 2 diabetic Goto-Kakizaki and normal Wistar rats. Nutr. Res. Pract. 2009, 3, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, L.; Batista, A.P.; Sousa, I.; Raymundo, A.; Bandarra, N.M. Microalgae in novel food products. Food Chem. Res. Dev. 2008, 3, 1–37. [Google Scholar]
- Queiroz, M.L.; Da Rocha, M.C.; Torello, C.; Souza Queiroz, J.; Bincoletto, C.; Morgano, M.A. Chlorella Vulgaris restores bone marrow cellularity and cytokine production in lead-exposed mice. Food. Chem. Toxicol. 2011, 49, 2934–2941. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Kim, I.; Kwon, S.H.; Kang, J.S.; Om, A.S. Protective effect of Chlorella Vulgaris against lead-induced oxidative stress in rat brains. J. Health. Sci. 2011, 57, 245–454. [Google Scholar] [CrossRef]
- Aizzat, O.; Yap, S.W.; Sopiah, H.; Madiha, M.M.; Hazreen, M.; Shailah, A.; Wan, J.W.; Nur, S.A.; Srijit, D.; Musalmah, M.; et al. Modulation of oxidative stress by Chlorella vulgaris in streptozotocin (STZ) induced diabetic Sprague-Dawley rats. Adv. Med. Sci. 2010, 55, 281–288. [Google Scholar] [CrossRef]
- Shibata, S.; Hayakawa, K.; Egashira, Y.; Sanada, H. Hypocholesterolemic mechanism of Chlorella: Chlorella and its indigestible fraction enhance hepatic cholesterol catabolism through up-regulation of cholesterol 7alpha-hydroxylase in rats. Biosci. Biotechnol. Biochem. 2007, 71, 916–925. [Google Scholar] [CrossRef]
- Vijayavel, K.; Anbuselvam, C.; Balasubramanian, M.P. Antioxidant effect of the marine algae Chlorella vulgaris against naphthalene-induced oxidative stress in the albino rats. Mol. Cell. Biochem. 2007, 303, 39–44. [Google Scholar] [CrossRef]
- Sibi, G.; Rabina, S. Inhibition of Pro-inflammatory mediators and cytokines by Chlorella Vulgaris extracts. Pharmacognosy Res. 2016, 8, 118–122. [Google Scholar] [CrossRef]
- Shih, M.F.; Chen, L.C.; Cherng, J.Y. Chlorella 11-peptide inhibits the production of macrophage-induced adhesion molecules and reduces endothelin-1 expression and endothelial permeability. Mar. Drugs 2013, 11, 3861–3877. [Google Scholar] [CrossRef] [PubMed]
- Renju, G.L.; Muraleedhara Kurup, G.; Saritha Kumari, C.H. Effect of lycopene from Chlorella marina on high cholesterol induced oxidative damage and inflammation in rats. Inflammopharmacology 2014, 22, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Shrafi, A.; Ebrahimi Mamaghani, S.; Kakaei, F.; Javadzadeh, Y.; Asghari Jafarabadi, M. The Effect of complex Chlorella Vulgaris microlevel on inflammatory factors in patients with non-alcoholic fatty liver: A double-sided clinical trial. J. Mazandaran Univ. Med. Sci. 2014, 24, 113–121. [Google Scholar]
- Suzuki, Y.; Kovacs, C.S.; Takanaga, H.; Peng, J.B.; Landowski, C.P.; Hediger, M.A. Calcium channel TRPV6 is involved in murine maternal-fetal calcium transport. J. Bone Miner. Res. 2008, 23, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Lee, G.S.; Yoo, Y.M.; Choi, K.C.; Jeung, E.B. Sodium/potassium/calcium exchanger 3 is regulated by the steroid hormones estrogen and progesterone in the uterus of mice during the estrous cycle. Biochem. Biophys. Res. Commun. 2009, 385, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Nijenhuis, T.; Hoenderop, J.G.; van der Kemp, A.W.; Bindels, R.J. Localization and regulation of the epithelial Ca2+ channel TRPV6 in the kidney. J. Am. Soc. Nephrol. 2003, 14, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, X.L.; Leung, P.C.; Che, C.T.; Wong, M.S. Fructus ligustri lucidi extract improves calcium balance and modulates the calciotropic hormone level and vitamin D-dependent gene expression in aged ovariectomized rats. Menopause 2008, 15, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. Sep. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Busse, B.; Djonic, D.; Milovanovic, P.; Hahn, M.; Püschel, K.; Ritchie, R.O.; Djuric, M.; Amling, M. Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell 2010, 9, 1065–1075. [Google Scholar] [CrossRef]
- Currey, J.D.; Shahar, R. Cavities in the compact bone in tetrapods and fish and their effect on mechanical properties. J. Struct. Biol. 2013, 183, 107–122. [Google Scholar] [CrossRef]
- Gourion-Arsiquaud, S.; Burket, J.C.; Havill, L.M.; DiCarlo, E.; Doty, S.B.; Mendelsohn, R.; Van Der Meulen, M.C.H.; Boskey, A.L. Spatial variation in osteonal bone properties relative to tissue and animal age. J. Bone Miner. Res. 2009, 24, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Hannah, K.M.; Thomas, C.D.L.; Clement, J.G.; De Carlo, F.; Peele, A.G. Bimodal distribution of osteocyte lacunar size in the human femoral cortex as revealed by micro-CT. Bone 2010, 47, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Nalla, R.K.; Kruzic, J.J.; Kinney, J.H.; Balooch, M.; Ager, J.W.; Ritchie, R.O. Role of microstructure in the aging-related deterioration of the toughness of human cortical bone. Mater. Sci. Eng. 2006, C 26, 1251–1260. [Google Scholar] [CrossRef]
- Pfeiffer, S.; Crowder, C.; Harrington, L.; Brown, M. Secondary osteon and Haversian canal dimensions as behavioral indicators. Am. J. Phys. Anthropol. 2006, 131, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Seeman, E.; Delmas, P.D. Bone quality―The material and structural basis of bone strength and fragility. N. Engl. J. Med. 2006, 354, 2250–2261. [Google Scholar] [CrossRef] [PubMed]
- Van Oers, R.F.M.; Ruimerman, R.; Tanck, E.; Hilbers, P.A.J.; Huiskes, R. A unified theory for osteonal and hemi-osteonal remodeling. Bone 2008, 42, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Bronner, F. Mechanisms and functional aspects of intestional calcium absorption. J. Exp. Zool. A Comp. Exp. Biol. 2003, 300A, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Asagiri, M.; Takayanagi, H. The molecular understanding of osteoclast differentiation. Bone 2007, 40, 251–264. [Google Scholar] [CrossRef]
- Whisner, C.M.; Martin, B.R.; Nakatsu, C.H.; McCabe, G.P.; McCabe, L.D.; Peacock, M.; Weaver, C.M. Soluble maize fibre affects short-term calcium absorption in adolescent boys and girls: A randomised controlled trial using dual stable isotopic tracers. Br. J. Nutr. 2014, 112, 446–456. [Google Scholar] [CrossRef]
- Zhao, C.; Yang, C.F.; Chen, M.J.; Lv, X.C.; Liu, B.; Yi, L.Z.; Cornara, L.; Wei, M.C.; Yang, Y.C.; Tundis, R.; et al. Regulatory efficacy of brown seaweed lessonia nigrescens extract on the gene expression profile and intestinal microflora in type 2 diabetic mice. Mol. Nutr. Food Res. 2018, 62, 1700730. [Google Scholar] [CrossRef]
- Cai, X.X.; Lin, J.P.; Wang, S.Y. Novel peptide with specific calcium-binding capacity from Schizochytrium sp. protein hydrolysates and calcium bioavailability in Caco-2 Cells. Mar. Drugs 2017, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Hua, P.P.; Yu, Z.Y.; Xiong, Y.; Liu, B.; Zhao, L.N. Regulatory efficacy of Spirulina platensis protease hydrolyzate on lipid metabolism and gut microbiota in high-fat diet-fed rats. J. Mol. Sci. 2018, 19, 4023. [Google Scholar] [CrossRef] [PubMed]
- Siping, X.; Xiao, Y.; Xiaofei, Y. Immunization with Na+/K+ ATPase DR peptide prevents bone loss in an ovariectomized rat osteoporosis mode. Biochem. Pharmacol. 2018, 23, 223–225. [Google Scholar]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.Z.; Li, T.T.; Liu, D.; Chen, Y.H.; Liu, Y.Y.; Liu, B.; Zhang, H.Y.; Zhao, C. Effect of marine microalga Chlorella pyrenoidosa ethanol extract on lipid metabolism and gut microbiota composition in high-fat diet-fed rats. Mar. Drugs 2018, 16, 498. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.L.; Zhao, L.N.; Cai, X.X.; Wang, S.Y.; Huang, Y.F.; Hong, J.; Rao, P.F. Purification and characterisation of a glutamic acid-containing peptide with calcium-binding capacity from whey protein hydrolysate. J. Dairy Res. 2015, 82, 29–35. [Google Scholar] [CrossRef]
- Liu, F.R.; Wang, L.; Wang, R.; Chen, Z.X. Calcium-binding capacity of wheat germ protein hydrolysate and characterization of peptide-calcium complex. J. Agric. Food Chem. 2013, 61, 7537–7544. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, K.; Yang, X.D.; Wang, L.L.; Shen, R.F. Complexation of Al(III) with reduced glutathione in acidic aqueous solutions. J. Inorg. Biochem. 2009, 103, 657–665. [Google Scholar] [CrossRef]
- Centeno, V.; Diaz de Barboza, G.; Marchionatti, A.; Rodriguez, V.; Tolosa de Talamoni, N. Molecular mechanisms triggered by low-calcium diets. Nutr. Res. Rev. 2009, 22, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Lv, Y.; Yang, B.C.; Guo, S.T. Aggregation of hydrophobic soybean protein hydrolysates: Changes in molecular weight distribution during storage. LWT Food Sci. Techonol. 2009, 42, 914–917. [Google Scholar] [CrossRef]
- Liu, H.; Xu, J.; Guo, S.T. Soybean peptide aggregates improved calcium binding capacity. LWT Food Sci. Techonol. 2016, 3, 174–180. [Google Scholar] [CrossRef]
- Yang, H.; Ahn, C.; Jeung, E.B. Differential expression of calcium transport genes caused by COMT inhibition in the duodenum, kidney and placenta of pregnant mice. Mol. Cell. Endocrinol. 2015, 401, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.; Heaney, R.P. Co-dependence of calcium and phosphorus for growth and bone development under conditions of varying deficiency. Bone 2003, 32, 532–540. [Google Scholar] [CrossRef]
- Robison, R.; Macleod, M.; Rosenheim, A.H. The possible significance of hexosephosphoric esters in ossification. Biochem. J. 1923, 17, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.C.; Chen, S.Y.; Liu, R.; Wu, H. A novel hydrolytic product from flesh of mactra veneriformis and its bioactivities in calcium supplement. J. Ocean Uni. China 2012, 11, 389–396. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.Y.; Ding, L.; Damaolar, A.; Li, Z.Q.; Qiu, Y.S.; Yin, Z.H. Lentivirus-TAZ administration alleviates osteoporotic phenotypes in the femoral neck of ovariectomized rats. Cell. Physiol. Biochem. 2016, 38, 283–294. [Google Scholar]
- Applegate, T.J.; Lilburn, M.S. Growth of the femur and tibia of a commercial broiler line. Poul. Sci. 2002, 81, 1289–1294. [Google Scholar] [CrossRef]
- Mora-Gutierrez, A.; Farrell, H.M.J.; Attaie, R. Effects of bovine and caprine Monterey Jack cheeses fortified with milk calcium on bone mineralization in rats. Int. Dairy J. 2007, 17, 255–267. [Google Scholar] [CrossRef]
- Stewart, A.F.; Cain, R.L.; Burr, D.B.; Jacob, D.; Turner, C.H.; Hock, J.M. Six-month daily administration of parathyroid hormone and parathyroid hormone-related protein peptides to adult ovariectomized rats markedly enhances bone mass and biomechanical properties: A comparison of human parathyroid hormon 1-34, parathyroid hormone-related protein 1-36, and SDZ-parathyroid hormone 893. J. Bone Miner Res. 2000, 15, 1517–1525. [Google Scholar]
- Lieben, L.; Benn, B.S.; Ajibade, D.; Stockmans, I.; Moermans, K.; Hediger, M.A. Trpv6 mediates intestinal calcium absorption during calcium restrictionn and contributes to bone homeostasis. Bone 2010, 47, 301–308. [Google Scholar] [CrossRef]
- Hanzlik, R.P.; Fowler, S.C.; Fisher, D.H. Relative bioavailability of calcium from calcium formate, calcium citrate, and calcium carbonate. J. Pharmacol. Exp. Ther. 2005, 313, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Titi, L.; Zemin, X.; Fei, C. Theabrownin suppresses, in vitro, osteoclastogenesis and prevents bone loss in ovariectomized rats. Biomed. Pharmacother. 2018, 106, 1339–1347. [Google Scholar]
- Mentaverri, R.; Yano, S.; Chattopadhyay, N.; Petit, L.; Kifor, O.; Kamel, S.; Terwilliger, E.F.; Brazier, M.; Brown, E.M. The calcium sensing receptor is directly involved in both osteoclast differentiation and apoptosis. Faseb J. 2006, 20, 2562–2564. [Google Scholar] [CrossRef] [PubMed]
- Kellett, G.L. Alternative perspective on intestinal calcium absorption: Proposed complementary actions of Ca(v)1.3 and TRPV6. Nutr. Rev. 2011, 69, 347–370. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Kim, Y.S.; Hwang, J.W.; Kim, E.K.; Moon, S.H.; Jeon, B.T.; Jeon, Y.J.; Kim, J.M.; Park, P.J. Purification and characterization of a novel antioxidative peptide from duck skin byproducts that protects liver against oxidative damage. Food Res. Int. 2012, 49, 285–295. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Sun, D. Short-term hypoxia accelerates bone loss in ovariectomized rats by suppressing osteoblastogenesis but enhancing osteoclastogenesis. Med. Sci. Monit. 2016, 22, 2962–2971. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lazarenko, O.P.; Kang, J. Feeding blueberry diets to young rats dose-dependently inhibits bone resorption through suppression of RANKL in stromal cells. PLoS ONE 2013, 8, 78–89. [Google Scholar] [CrossRef]
- Nilsson, A.G.; Sundh, D.; Bäckhed, F.; Lorentzon, M. Lactobacillus reuteri reduces bone loss in older women with low bone mineral density: A randomized, placebo-controlled, double-blind, clinical trial. J. Intern. Med. 2018, 284, 307–317. [Google Scholar] [CrossRef]
- Parvaneh, K.; Ebrahimi, M.; Sabran, M.R. Probiotics (Bifidobacterium longum) increase bone mass density and upregulate Sparc and Bmp-2 genes in rats with bone loss resulting from ovariectomy. Bio. Med. Res. Int. 2015, 2015, 1–10. [Google Scholar]
- Britton, R.A.; Irwin, R.; Quach, D. Probiotic L. reuteri treatment prevents bone loss in a menopausal ovariectomized mouse model. J. Cell. Physiol. 2014, 229, 1822–1830. [Google Scholar] [CrossRef]
- Ohlsson, C.; Engdahl, C.; Fåk, F. Probiotics protect mice from ovariectomyinduced cortical bone loss. PLoS ONE 2014, 9, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Amdekar, S.; Singh, V.; Singh, R. Lactobacillus casei reduces the inflammatory joint damage associated with collagen-induced arthritis (CIA) by reducing the pro-inflammatory cytokines: Lactobacillus casei: COX-2 inhibitor. J. Clin. Immunol. 2011, 31, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wu, L.F.; Wang, W.Y.; Lu, X.; Jiang, Z.H.; Zhang, Y.H.; Jiang, D.H.; Jiang, J.N.; Gao, H.Q.; Lei, S.F.; et al. Anxa2 attenuates osteoblast growth and is associated with hip BMD and osteoporotic fracture in Chinese elderly. PLoS ONE 2018, 13, e0194781. [Google Scholar] [CrossRef] [PubMed]
- Nishide, Y.; Tadaishi, M.; Kobori, M. Possible role of S-equol on bone loss via amelioration of inflammatory indices in ovariectomized mice. J. Clin. Biochem. Nutr. 2013, 53, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nima, M.N.; Younes, G.; Hossein, D.M. Supportive role of probiotic strains in protecting rats from ovariectomy-induced cortical bone loss. Probiotics Antimicrob. Proteins 2018, 65, 34–38. [Google Scholar]
- Saltzman, J.R.; Russell, R.M. The aging gut. Nutritional issues. Gastroenterol Clin. North Am. 1998, 27, 309–324. [Google Scholar] [CrossRef]
- Schwarzer, M.; Makki, K.; Storelli, G.; Machuca-Gayet, I.; Srutkova, D.; Hermanova, P.; Martino, M.E.; Balmand, S.; Hudcovic, T.; Heddi, A.; et al. Lactobacillus plantarum strain maintains growth of infant mice during chronic undernutrition. Science 2016, 351, 854–857. [Google Scholar] [CrossRef]
- Yan, J.; Herzog, J.W.; Tsang, K.; Brennan, C.A.; Bower, M.A.; Garrett, W.S.; Sartor, B.R.; Aliprantis, A.O.; Charles, J.F. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc. Natl. Acad. Sci. USA 2016, 113, E7554–E7563. [Google Scholar] [CrossRef]
- Bryk, G.; Coronel, M.Z.; Pellegrini, G. Effect of a combination GOS/FOS prebiotic mixture and interaction with calcium intake on mineral absorption and bone parameters in growing rats. Eur. J. Nutr. 2015, 54, 913–923. [Google Scholar] [CrossRef]
- Fonseca, D.; Ward, W.E. Daidzein together with high calcium preserve bone mass and biomechanical strength at multiple sites in ovariectomized mice. Bone 2004, 35, 489–497. [Google Scholar] [CrossRef]
- Rodrigues, F.C.; Castro, A.S.B.; Rodrigues, V.C. Yacon flour and Bifidobacterium longum modulate bone health in rats. J. Med. Food. 2012, 15, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Conesa, D.; López, G.; Abellán, P.; Ros, G. Bioavailability of calcium, magnesium and phosphorus in rats fed probiotic, prebiotic and synbiotic powder follow-up infant formulas and their effect on physiological and nutritional parameters. J. Sci. Food Agric. 2006, 86, 2327–2336. [Google Scholar] [CrossRef]
- Gilman, J.; Cashman, K.D. The effect of probiotic bacteria on transepithelial calcium transport and calcium uptake in human intestinal-like Caco-2 cells. Curr. Issues Intest. Microbiol. 2006, 7, 1–5. [Google Scholar] [PubMed]








| Groups | Weight (g) | |||
|---|---|---|---|---|
| 0 Weeks | 4 Weeks | 8 Weeks | Weight Gain | |
| Control | 120.04 ± 4.66a | 303.62 ± 10.43a | 371.21 ± 14.01a | 245.13 ± 9.00a |
| Model | 125.19 ± 4.79a | 267.32 ± 5.62b | 308.56 ± 4.58e | 189.36 ± 8.33f |
| HCaCO3 | 120.39 ± 9.22a | 269.63 ± 14.27b | 326.33 ± 19.47bcd | 211.42 ± 9.63bcd |
| HGCa | 122.70 ± 2.19a | 271.54 ± 13.35b | 331.92 ± 11.43bc | 221.79 ± 10.96bc |
| LCPPH + LCaCO3 | 122.443 ± 4.75a | 266.95 ± 11.27b | 320.97 ± 14.36cde | 196.53 ± 11.53ef |
| MCPPH + MCaCO3 | 119.72 ± 5.43a | 271.54 ± 17.60b | 328.01 ± 24.35bcd | 208.23 ± 21.50cde |
| HCPPH + HCaCO3 | 123.94 ± 4.76a | 269.85 ± 12.52b | 333.47 ± 17.22bc | 214.70 ± 11.34bcd |
| LCPPH-Ca | 123.34 ± 4.49a | 267.35 ± 9.50b | 325.72 ± 20.89bcd | 202.38 ± 17.97def |
| MCPPH-Ca | 121.65 ± 4.63a | 269.20 ± 6.67b | 332.21 ± 11.45bc | 204.33 ± 15.22de |
| HCPPH-Ca | 121.85 ± 7.19a | 270.62 ± 13.87b | 340.02 ± 20.01a | 223.41 ± 13.03b |
| No. | Group | n | Given Dosage of N Content and Ca, mg/kg bw |
|---|---|---|---|
| 1 | Control | 10 | Deionized water |
| 2 | Model | 10 | Deionized water |
| 3 | HCaCO3 | 10 | Ca 119.97 |
| 4 | HGCa | 10 | Ca 119.97 |
| 5 | LCPPH + LCaCO3 | 10 | CPPH 465 + Ca 39.99 |
| 6 | MCPPPH + MCaCO3 | 10 | CPPH 930 + Ca 79.98 |
| 7 | HCPPH + HCaCO3 | 10 | CPPH1395 + Ca 119.97 |
| 8 | LCPPH-Ca | 10 | Ca 39.99 |
| 9 | MCPPH-Ca | 10 | Ca 79.98 |
| 10 | HCPPH-Ca | 10 | Ca 119.97 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, P.; Xiong, Y.; Yu, Z.; Liu, B.; Zhao, L. Effect of Chlorella Pyrenoidosa Protein Hydrolysate-Calcium Chelate on Calcium Absorption Metabolism and Gut Microbiota Composition in Low-Calcium Diet-Fed Rats. Mar. Drugs 2019, 17, 348. https://doi.org/10.3390/md17060348
Hua P, Xiong Y, Yu Z, Liu B, Zhao L. Effect of Chlorella Pyrenoidosa Protein Hydrolysate-Calcium Chelate on Calcium Absorption Metabolism and Gut Microbiota Composition in Low-Calcium Diet-Fed Rats. Marine Drugs. 2019; 17(6):348. https://doi.org/10.3390/md17060348
Chicago/Turabian StyleHua, Pengpeng, Yu Xiong, Zhiying Yu, Bin Liu, and Lina Zhao. 2019. "Effect of Chlorella Pyrenoidosa Protein Hydrolysate-Calcium Chelate on Calcium Absorption Metabolism and Gut Microbiota Composition in Low-Calcium Diet-Fed Rats" Marine Drugs 17, no. 6: 348. https://doi.org/10.3390/md17060348
APA StyleHua, P., Xiong, Y., Yu, Z., Liu, B., & Zhao, L. (2019). Effect of Chlorella Pyrenoidosa Protein Hydrolysate-Calcium Chelate on Calcium Absorption Metabolism and Gut Microbiota Composition in Low-Calcium Diet-Fed Rats. Marine Drugs, 17(6), 348. https://doi.org/10.3390/md17060348