Pinnatoxins’ Deleterious Effects on Cholinergic Networks: From Experimental Models to Human Health
Abstract
:1. Introduction
1.1. Ecological Aspects
1.2. Chemistry of PnTXs
2. Acute Toxicity
2.1. In Vivo: Symptoms Observed in Mice
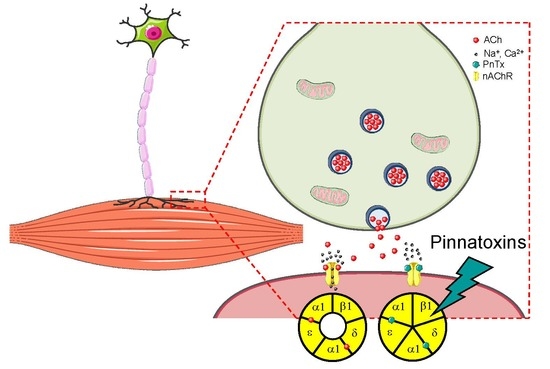
2.2. In Vitro Muscle Paralysis
2.3. In Vitro: Molecular and Cellular Targets
2.3.1. Nicotinic Acetylcholine Receptors (nAChRs)
Muscle nAChRs
Neuronal nAChRs
2.3.2. PnTXs Target nAChRs
3. Possible Effects to Humans by Extrapolation of Experimental Data
3.1. Clinical Effects of nAChR Antagonists
3.1.1. Skeletal Muscle Relaxants
3.1.2. Autonomic Agents
3.2. Human Diseases and Pntx Exposure: Autonomic Dysfunctions?
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zheng, S.; Huang, F.; Chen, S.; Tan, X.; Zuo, J.; Peng, J.; Xie, R. The isolation and bioactivities of pinnatoxin. Chin. J. Mar. Drugs 1990, 9, 33–35. [Google Scholar]
- Uemura, D.; Chou, T.; Haino, T.; Nagatsu, A.; Fukuzawa, S.; Zheng, S.-Z.; Chen, H.-S. Pinnatoxin A: a toxic amphoteric macrocycle from the Okinawan bivalve Pinna muricata. J. Am. Chem. Soc. 1995, 117, 1155–1156. [Google Scholar] [CrossRef]
- Rhodes, L.; Smith, K.; Selwood, A.; McNabb, P.; Van Ginkel, R.; Holland, P.; Munday, R. Production of pinnatoxins by a peridinoid dinoflagellate isolated from Northland, New Zealand. Harmful Algae 2010, 9, 384–389. [Google Scholar] [CrossRef]
- Molgó, J.; Marchot, P.; Aráoz, R.; Benoit, E.; Iorga, B.I.; Zakarian, A.; Taylor, P.; Bourne, Y.; Servent, D. Cyclic imine toxins from dinoflagellates: A growing family of potent antagonists of the nicotinic acetylcholine receptors. J. Neurochem. 2017, 142, 41–51. [Google Scholar] [CrossRef]
- Nézan, E.; Chomérat, N. Vulcanodinium rugosum gen. nov., sp. nov. (dinophyceae): A new marine dinoflagellate from the French Mediterranean coast. Cryptogam. Algol. 2011, 32, 3–18. [Google Scholar] [CrossRef]
- Hess, P.; Abadie, E.; Hervé, F.; Berteaux, T.; Séchet, V.; Araoz, R.; Molgó, J.; Zakarian, A.; Sibat, M.; Rundberget, T.; et al. Pinnatoxin G is responsible for atypical toxicity in mussels (Mytilus galloprovincialis) and clams (Venerupis decussata) from Ingril, a French Mediterranean lagoon. Toxicon 2013, 75, 16–26. [Google Scholar] [CrossRef]
- Rossini, G.P.; Hess, P. Phycotoxins: Chemistry, mechanisms of action and shellfish poisoning. Exp. Suppl. 2010, 100, 65–122. [Google Scholar]
- Selwood, A.I.; Miles, C.O.; Wilkins, A.L.; Van Ginkel, R.; Munday, R.; Rise, F.; McNabb, P. Isolation, structural determination and acute toxicity of pinnatoxins E, F and G. J. Agric. Food Chem. 2010, 58, 6532–6542. [Google Scholar] [CrossRef]
- McCarron, P.; Rourke, W.A.; Hardstaff, W.; Pooley, B.; Quilliam, M.A. Identification of pinnatoxins and discovery of their fatty acid ester metabolites in mussels (Mytilus edulis) from Eastern Canada. J. Agric. Food Chem. 2012, 60, 1437–1446. [Google Scholar] [CrossRef]
- Takada, N.; Umemura, N.; Suenaga, K.; Uemura, D. Structural determination of pteriatoxins A, B and C, extremely potent toxins from the bivalve Pteria penguin. Tetrahedron Lett. 2001, 42, 3495–3497. [Google Scholar] [CrossRef]
- Munday, R.; Selwood, A.I.; Rhodes, L. Acute toxicity of pinnatoxins E, F and G to mice. Toxicon 2012, 60, 995–999. [Google Scholar] [CrossRef]
- Fessard, V.; Huguet, A.; Sosa, S.; Tubaro, A.; Aráoz, R.; Molgó, J. Pinnatoxines en lien avec l’espèce Vulcanodinium rugosum. Final report (72p). 2014. Available online: https://archimer.ifremer.fr/doc/00285/39635/38127.pdf (accessed on 19 July 2019).
- Selwood, A.I.; Wilkins, A.L.; Munday, R.; Gu, H.; Smith, K.F.; Rhodes, L.L.; Rise, F. Pinnatoxin H: A new pinnatoxin analogue from a South China Sea Vulcanodinium rugosum isolate. Tetrahedron Lett. 2014, 55, 5508–5510. [Google Scholar] [CrossRef]
- Molgó, J.; Institut des Neurosciences Paris-Saclay, Centre National de la Recherche Scientifique (CNRS), UMR 9197 CNRS/Université Paris-Sud, F-91198 Gif-sur-Yvette, France. Personal communication, 2019.
- Benoit, E.; Couesnon, A.; Lindovsky, J.; Iorga, B.I.; Aráoz, R.; Servent, D.; Zakarian, A.; Molgó, J. Synthetic pinnatoxins A and G reversibly block mouse skeletal neuromuscular transmission in vivo and in vitro. Mar. Drugs 2019, 17, 306. [Google Scholar] [CrossRef]
- Hellyer, S.D.; Selwood, A.I.; Rhodes, L.; Kerr, D.S. Marine algal pinnatoxins E and F cause neuromuscular block in an in vitro hemidiaphragm preparation. Toxicon 2011, 58, 693–699. [Google Scholar] [CrossRef]
- Hellyer, S.D.; Selwood, A.I.; Rhodes, L.; Kerr, D.S. Neuromuscular blocking activity of pinnatoxins E, F and G. Toxicon 2013, 76, 214–220. [Google Scholar] [CrossRef]
- Couesnon, A.; Lindovsky, J.; Zakarian, A.; Creuzet, S.; Molgo, J. Pinnatoxins block skeletal neuromuscular junction activity and affect embryo development. Toxicon 2014, 91, 175–176. [Google Scholar] [CrossRef]
- Gotti, C.; Zoli, M.; Clementi, F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol. Sci. 2006, 27, 482–491. [Google Scholar] [CrossRef]
- Zoli, M.; Pucci, S.; Vilella, A.; Gotti, C. Neuronal and extraneuronal nicotinic acetylcholine receptors. Curr. Neuropharmacol. 2018, 16, 338–349. [Google Scholar] [CrossRef]
- Dajas-Bailador, F.; Wonnacott, S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol. Sci. 2004, 25, 317–324. [Google Scholar] [CrossRef]
- Collingridge, G.L.; Olsen, R.W.; Peters, J.; Spedding, M. A nomenclature for ligand-gated ion channels. Neuropharmacology 2009, 56, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J.B.; Weber, M.; Huchet, M.; Changeux, J.P. Purification from Torpedo marmorata electric tissue of membrane fragments particularly rich in cholinergic receptor protein. FEBS Lett. 1972, 26, 43–47. [Google Scholar] [CrossRef]
- Grassi, F.; Fucile, S. Calcium influx through muscle nAChR-channels: one route, multiple roles. Neuroscience 2019, in press. [Google Scholar] [CrossRef]
- George, A.L., Jr.; Komisarof, J.; Kallen, R.G.; Barchi, R.L. Primary structure of the adult human skeletal muscle voltage-dependent sodium channel. Ann. Neurol. 1992, 31, 131–137. [Google Scholar] [CrossRef]
- Araoz, R.; Servent, D.; Molgó, J.; Iorga, B.I.; Fruchart-Gaillard, C.; Benoit, E.; Gu, Z.; Stivala, C.; Zakarian, A. Total synthesis of pinnatoxins A and G and revision of the mode of action of pinnatoxin A. J. Am. Chem. Soc. 2011, 133, 10499–10511. [Google Scholar] [CrossRef]
- Bourne, Y.; Sulzenbacher, G.; Radić, Z.; Aráoz, R.; Reynaud, M.; Benoit, E.; Zakarian, A.; Servent, D.; Molgó, J.; Taylor, P.; et al. Marine macrocyclic imines, pinnatoxins A and G: structural determinants and functional properties to distinguish neuronal α7 from muscle α1(2)βγδ nAChRs. Structure 2015, 23, 1106–1115. [Google Scholar] [CrossRef]
- Moreira, A.R.; Comas, A.; Valle, A.; Seisdedo, M.; Fernandes, L.F. Bloom of Vulcanodinium rugosum linked to skin lesions in Cienfuegos Bay, Cuba. Harmful Algae News 2016, 55, 10–11. [Google Scholar]
- Geiger, M.; Desanglois, G.; Hogeveen, K.; Fessard, V.; Leprêtre, T.; Mondeguer, F.; Guitton, Y.; Herve, F.; Séchet, V.; Grovel, O.; et al. Cytotoxicity, fractionation and dereplication of extracts of the dinoflagellate Vulcanodinium rugosum, a producer of pinnatoxin G. Mar. Drugs 2013, 11, 3350–3371. [Google Scholar] [CrossRef]
- Grando, S.A.; Horton, R.M.; Pereira, E.F.R.; Diethelm-Okita, B.M.; George, P.M.; Albuquerque, E.X.; Conti-Fine, B.M. A nicotinic acetylcholine receptor regulating cell adhesion and motility is expressed in human keratinocytes. J. Investig. Dermatol. 1995, 105, 774–781. [Google Scholar] [CrossRef]
- Bowman, W.C. Neuromuscular block. Br. J. Pharmacol. 2006, 147, S277–S286. [Google Scholar] [CrossRef]
- Wehrwein, E.A.; Orer, H.S.; Barman, S.M. Overview of the anatomy, physiology, and pharmacology of the autonomic nervous system. Compr. Physiol. 2016, 6, 1239–1278. [Google Scholar]
- Becker, D.E. Basic and clinical pharmacology of autonomic drugs. Anesthesia Prog. 2012, 59, 159–169. [Google Scholar] [CrossRef]
- Senanayake, N.; Roman, G.C. Disorders of neuromuscular transmission due to natural environmental toxins. J. Neurol. Sci. 1992, 107, 1–13. [Google Scholar] [CrossRef]
- Young, J. Mecamylamine: New therapeutic uses and toxicity/risk profile. Clin. Ther. 2001, 23, 532–565. [Google Scholar] [CrossRef]
- Litkey, J.; Dailey, M.W. Anticholinergic toxicity associated with the ingestion of lupini beans. Am. J. Emerg. Med. 2007, 25, 215–217. [Google Scholar] [CrossRef]
- Ortega, J.A.; Lazerson, J. Anagyrine-induced red cell aplasia, vascular anomaly, and skeletal dysplasia. J. Pediatr. 1987, 111, 87–89. [Google Scholar] [CrossRef]
- Panter, K.E.; James, L.F.; Gardner, D.R. Lupines, poison-hemlock and Nicotiana spp.: toxicity and teratogenicity in livestock. J. Nat. Toxins 1999, 8, 117–134. [Google Scholar]
- Hurst, R.; Rollema, H.; Bertrand, D. Nicotinic acetylcholine receptors: from basic science to therapeutics. Pharmacol. Ther. 2013, 137, 22–54. [Google Scholar] [CrossRef]
- Ito, Y.; Miledi, R.; Molenaar, P.C.; Vincent, A.; Polak, R.L.; van Gelder, M.; Davis, J.N. Acetylcholine in human muscle. Proc. R. Soc. Lond. B Biol. Sci. 1976, 192, 475–480, PMID: 4804. [Google Scholar]
- Vincent, A.; Newsom-Davis, J.; Martin, V. Anti-acetylcholine receptor antibodies in d-penicillamine-associated Myasthenia Gravis. Lancet 1978, 311, 1254. [Google Scholar] [CrossRef]
- Heinemann, S.; Merlie, J.; Lindström, J. Modulation of acetylcholine receptor in rat diaphragm by anti-receptor sera. Nature 1978, 274, 65–68. [Google Scholar] [CrossRef]
- Lindstrom, J. How the autoimmune response to acetylcholine receptor impairs neuromuscular transmission in myasthenia gravis and its animal model. Fed. Proc. 1978, 37, 2828–2830. [Google Scholar]
- Engstrom, J.W. Myasthenia Gravis: Diagnostic Mimics. Semin. Neurol. 2004, 24, 141–147. [Google Scholar] [CrossRef]
- Scherer, K.; Bedlack, R.S.; Simel, D.L. Does this patient have myasthenia gravis? JAMA 2005, 293, 1906. [Google Scholar] [CrossRef]
- Nguyen-Huu, T.; Molgó, J.; Servent, D.; Duvaldestin, P. Resistance to D-tubocurarine of the rat diaphragm as compared to a limb muscle: influence of quantal transmitter release and nicotinic acetylcholine receptors. Anesthesiology 2009, 110, 1011–1015. [Google Scholar] [CrossRef]
- Lamas, J.P.; Arévalo, F.; Moroño, Á.; Correa, J.; Muñíz, S.; Blanco, J. Detection and spatio-temporal distribution of pinnatoxins in shellfish from the Atlantic and Cantabrian coasts of Spain. Toxins 2019, 11, 340. [Google Scholar] [CrossRef]
- Ajani, P.; Harwood, D.T.; Murray, S.A. Recent trends in marine phycotoxins from Australian coastal waters. Mar. Drugs 2017, 15, 33. [Google Scholar] [CrossRef]
- ANSES. Opinion of the French Agency for Food, Environmental and Occupational Health & Safety on the Assessment of the Health Risks Associated with Pinnatoxins in Shellfish. 2019. Available online: https://www.anses.fr/en/system/files/ERCA2016SA0013EN.pdf (accessed on 19 July 2019).


| Toxin and % Purity | Route of Administration and Conditions | LD50 (µg/kg pc) | MTD (µg/kg pc) | References |
|---|---|---|---|---|
| PnTX E* | Per os | 2800 | 600 | [11] |
| Fed mice | CI95: 2380–3000 | |||
| PnTX F* | Per os | 25.0 | 9,9 | [11] |
| Fed mice | CI95: 19.1–35.1 | |||
| Per os | 29.9 | ND | ||
| 16 h fasted mice | CI95: 25–32 | |||
| Voluntary intake | 50.0 | 16,0 | ||
| Fed mice | CI95: 39.4–62.8 | |||
| Voluntary intake | 50.0 | ND | ||
| Fed mice | CI95: 37.9–71.5 | |||
| Voluntary intake | 50.0 | ND | ||
| 16 h fasted mice | CI95:37.9–71.5 | |||
| Voluntary intake | 77.0 | ND | ||
| 16h fasted mice | CI95: ND | |||
| Voluntary intake | 50.0 | ND | ||
| 16h fasted mice | CI95: 39.4–62.8 | |||
| PnTX-G* | Per os | 150.0 | 75 | [11] |
| Fed mice | CI95:105–100 | |||
| PnTX-G (100%) | Per os | 208.0 | 120 | [12] |
| 3 h fasted mice | CI95: 155–281 | |||
| PnTX-G* | Voluntary intake | 400.0 | 153 | [11] |
| Fed mice | CI95: 380–470 | |||
| PnTX H* | Per os | 163.0 | ND | [13] |
| CI95: 139–175 |
| Clinical Signs of Toxicity in Mice. | Anatomical or Physiological Support | Possible Outcome in Humans |
|---|---|---|
| Loss of motor activity | Neuromuscular junction impairment (skeletal muscles) | Myasthenic syndrome analogous with the disease myasthenia gravis Flaccid paralysis caused by curare |
| Respiratory depression/arrest | Neuromuscular junction impairment/block (diaphragm) | Respiratory impairment/arrest in myasthenia and myasthenic crisis |
| Seizure | Central damage via nAChR inhibition | Seizure crisis (impaired GABA release or hereditary mutations) |
| Leg extension | Spinal interneuron impairment Central impairment | Pyramidal syndrome Babinski sign |
| Reversibility if no death occurs or after prostigmine injection | Removal of the post-synaptic neuromuscular block | Fluctuation in the degree of myasthenic syndrome or temporary removal of the block with prostigmine Removal of the curare action |
| Exophthalmos | Increased intraocular pressure | Action of suxamethonium Action of lupin |
| Hypersalivation, vomiting, diarrhea, bradycardia, bronchoconstriction, miosis | Inhibition of neuronal communication at ganglia synapses | Autonomous agents acting on the sympathetic nervous system (side effects). Nicotinic syndrome |
| Tachycardia, blood hypertension, mydriasis | Inhibition of neuronal communication at ganglia synapses | Autonomous agents acting on the parasympathetic nervous system (side effects). Muscarinic syndrome |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delcourt, N.; Lagrange, E.; Abadie, E.; Fessard, V.; Frémy, J.-M.; Vernoux, J.-P.; Peyrat, M.-B.; Maignien, T.; Arnich, N.; Molgó, J.; et al. Pinnatoxins’ Deleterious Effects on Cholinergic Networks: From Experimental Models to Human Health. Mar. Drugs 2019, 17, 425. https://doi.org/10.3390/md17070425
Delcourt N, Lagrange E, Abadie E, Fessard V, Frémy J-M, Vernoux J-P, Peyrat M-B, Maignien T, Arnich N, Molgó J, et al. Pinnatoxins’ Deleterious Effects on Cholinergic Networks: From Experimental Models to Human Health. Marine Drugs. 2019; 17(7):425. https://doi.org/10.3390/md17070425
Chicago/Turabian StyleDelcourt, Nicolas, Emmeline Lagrange, Eric Abadie, Valérie Fessard, Jean-Marc Frémy, Jean-Paul Vernoux, Marie-Bénédicte Peyrat, Thomas Maignien, Nathalie Arnich, Jordi Molgó, and et al. 2019. "Pinnatoxins’ Deleterious Effects on Cholinergic Networks: From Experimental Models to Human Health" Marine Drugs 17, no. 7: 425. https://doi.org/10.3390/md17070425
APA StyleDelcourt, N., Lagrange, E., Abadie, E., Fessard, V., Frémy, J.-M., Vernoux, J.-P., Peyrat, M.-B., Maignien, T., Arnich, N., Molgó, J., & Mattei, C. (2019). Pinnatoxins’ Deleterious Effects on Cholinergic Networks: From Experimental Models to Human Health. Marine Drugs, 17(7), 425. https://doi.org/10.3390/md17070425