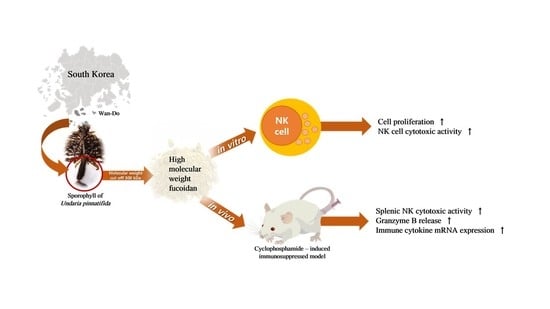
Characterization and Immunomodulatory Effects of High Molecular Weight Fucoidan Fraction from the Sporophyll of Undaria pinnatifida in Cyclophosphamide-Induced Immunosuppressed Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of the HMWF and LMWF
2.2. The molecular Weight of HMWF and LMWF
2.3. Effect of HMWF and LMWF on the Proliferation of NK-92MI Cells
2.4. Effect of HMWF and LMWF on NK Cell Cytotoxic Activity
2.5. Effect of HMWF and LMWF on Splenic NK Cell Cytotoxic Activity and Granzyme B Release in CY-Treated Mice
2.6. Effect of HMWF on Splenic Cytokine Gene Expression in the CY-Treated Mice
3. Materials and Methods
3.1. Materials
3.2. Extraction and Fractionation of Fucoidan Fraction (FF) into HMWF and LMWF from the Sporophyll of Undaria pinnatifida
3.3. Chemical Composition of HMWF and LMWF
3.3.1. Total Sugar Content
3.3.2. Uronic Acid Content
3.3.3. Sulfate Content
3.4. Determination of Monosaccharide Composition of HMWF and LMWF
3.5. Determination of Molecular Weight by HPGPC
3.6. In Vitro Assay
3.6.1. Cell Culture and Proliferation Assay
3.6.2. NK Cell Cytotoxic Activity Assay
3.7. In Vivo Assay
3.7.1. Treatment and Experimental Design
3.7.2. Splenic NK Cell Cytotoxic Activity Assay
3.7.3. Granzyme B Secretion and Quantification by ELISA
3.7.4. Quantitative Reverse Transcription-Polymerase Chain Reaction Assay
3.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prendergast, G.C.; Jaffee, E.M. Cancer immunologists and cancer biologists: Why we didn’t talk then but need to now. Cancer Res. 2007, 67, 3500–3504. [Google Scholar] [CrossRef] [PubMed]
- Ahlmann, M.; Hempel, G. The effect of cyclophosphamide on the immune system: Implications for clinical cancer therapy. Cancer Chemother. Pharmacol. 2016, 78, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.J. Clinical pharmacokinetics of cyclophosphamide. Clin. Pharmacokinet. 1991, 20, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Bracci, L.; Moschella, F.; Sestili, P.; La Sorsa, V.; Valentini, M.; Canini, I.; Baccarini, S.; Maccari, S.; Ramoni, C.; Belardelli, F. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin. Cancer Res. 2007, 13, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kim, M.; Kang, H.; Lee, H.; Park, Y.; Lee, Y.; Kang, Y.; Hwa, G.; Kang, Y.; Jung, Y. Immunomodulating properties of polygonum multiflorum extracts on cyclophosphamide-induced immunosuppression model. Indian J. Pharm. Sci. 2018, 80, 749–755. [Google Scholar] [CrossRef]
- Motoyoshi, Y.; Kaminoda, K.; Saitoh, O.; Hamasaki, K.; Nakao, K.; Ishii, N.; Nagayama, Y.; Eguchi, K. Different mechanisms for anti-tumor effects of low-and high-dose cyclophosphamide. Oncol. Rep. 2006, 16, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar] [CrossRef] [Green Version]
- Mak, W.; Hamid, N.; Liu, T.; Lu, J.; White, W. Fucoidan from New Zealand Undaria pinnatifida: Monthly variations and determination of antioxidant activities. Carbohydr. Polym. 2013, 95, 606–614. [Google Scholar] [CrossRef]
- Lee, J.-B.; Hayashi, K.; Hashimoto, M.; Nakano, T.; Hayashi, T. Novel antiviral fucoidan from sporophyll of Undaria pinnatifida (Mekabu). Chem. Pharm. Bull. 2004, 52, 1091–1094. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; Wang, J.; Ma, S.; Yu, Y.; White, W.L.; Yang, S.; Yang, F.; Lu, J. Fucoidan Extracted from Undaria pinnatifida: Source for Nutraceuticals/Functional Foods. Mar. Drugs 2018, 16, 321. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Shi, K.; Chen, S.; Wang, J.; Hassouna, A.; White, L.; Merien, F.; Xie, M.; Kong, Q.; Li, J. Fucoidan extracted from the New Zealand Undaria pinnatifida—Physicochemical comparison against five other fucoidans: Unique low molecular weight fraction bioactivity in breast cancer cell lines. Mar. Drugs 2018, 16, 461. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.-C.; Kim, W.-J.; Kim, S.-Y.; Kim, S.-M.; Chung, M.-K.; Park, J.-W.; Suh, H.-H.; Lee, K.-B.; Park, Y.-I. Immunomodulating activity of a fucoidan isolated from Korean Undaria pinnatifida sporophyll. Algae 2007, 22, 333–338. [Google Scholar] [CrossRef]
- Bi, D.; Yu, B.; Han, Q.; Lu, J.; White, W.L.; Lai, Q.; Cai, N.; Luo, W.; Gu, L.; Li, S. Immune activation of RAW264. 7 macrophages by low molecular weight fucoidan extracted from New Zealand Undaria pinnatifida. J. Agric. Food Chem. 2018, 66, 10721–10728. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Tamauchi, H.; Hashimoto, M.; Nakano, T. Antitumor activity and immune response of Mekabu fucoidan extracted from Sporophyll of Undaria pinnatifida. In Vivo 2003, 17, 245–249. [Google Scholar] [PubMed]
- Maruyama, H.; Tamauchi, H.; Iizuka, M.; Nakano, T. The role of NK cells in antitumor activity of dietary fucoidan from Undaria pinnatifida sporophylls (Mekabu). Planta Med. 2006, 72, 1415–1417. [Google Scholar] [CrossRef]
- Negishi, H.; Mori, M.; Mori, H.; Yamori, Y. Supplementation of elderly Japanese men and women with fucoidan from seaweed increases immune responses to seasonal influenza vaccination. J. Nutr. 2013, 143, 1794–1798. [Google Scholar] [CrossRef]
- Takai, M.; Miyazaki, Y.; Tachibana, H.; Yamada, K. The enhancing effect of fucoidan derived from Undaria pinnatifida on immunoglobulin production by mouse spleen lymphocytes. Biosci. Biotechnol. Biochem. 2014, 78, 1743–1747. [Google Scholar] [CrossRef]
- Choi, E.-M.; Kim, A.-J.; Kim, Y.-O.; Hwang, J.-K. Immunomodulating activity of arabinogalactan and fucoidan in vitro. J. Med. Food 2005, 8, 446–453. [Google Scholar] [CrossRef]
- Synytsya, A.; Kim, W.-J.; Kim, S.-M.; Pohl, R.; Synytsya, A.; Kvasnička, F.; Čopíková, J.; Park, Y.I. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef]
- Wijesinghe, W.; Jeon, Y.-J. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr. Polym. 2012, 88, 13–20. [Google Scholar] [CrossRef]
- Fonseca, R.J.; Oliveira, S.-N.M.; Melo, F.R.; Pereira, M.G.; Benevides, N.M.; Mourão, P.A. Slight differences in sulfation of algal galactans account for differences in their anticoagulant and venous antithrombotic activities. Thromb. Haemost. 2008, 99, 539–545. [Google Scholar] [CrossRef]
- Nishino, T.; Aizu, Y.; Nagumo, T. The influence of sulfate content and molecular weight of a fucan sulfate from the brown seaweed Ecklonia kurome on its antithrombin activity. Thrombos. Res. 1991, 64, 723–731. [Google Scholar] [CrossRef]
- Cho, M.L.; Lee, B.-Y.; You, S.G. Relationship between oversulfation and conformation of low and high molecular weight fucoidans and evaluation of their in vitro anticancer activity. Molecules 2011, 16, 291–297. [Google Scholar] [CrossRef]
- Yang, C.; Chung, D.; Shin, I.S.; Lee, H.; Kim, J.; Lee, Y.; You, S. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int. J. Biol. Macromol. 2008, 43, 433–437. [Google Scholar] [CrossRef]
- Rabinowich, H.; Sedlmayr, P.; Herberman, R.B.; Whiteside, T.L. Increased proliferation, lytic activity, and purity of human natural killer cells cocultured with mitogen-activated feeder cells. Cell. Immunol. 1991, 135, 454–470. [Google Scholar] [CrossRef]
- Warren, H.S. NK cell proliferation and inflammation. Immunol. Cell Biol. 1996, 74, 473–480. [Google Scholar] [CrossRef]
- Lieberman, J. Cell death and immunity: The ABCs of granule-mediated cytotoxicity: New weapons in the arsenal. Nat. Rev. Immun. 2003, 3, 361. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Surayot, U.; You, S. Structural effects of sulfated polysaccharides from Codium fragile on NK cell activation and cytotoxicity. Int. J. Biol. Macromol. 2017, 98, 117–124. [Google Scholar] [CrossRef]
- Lori, A.; Perrotta, M.; Lembo, G.; Carnevale, D. The spleen: A hub connecting nervous and immune systems in cardiovascular and metabolic diseases. Int. J. Mol. Sci. 2017, 18, 1216. [Google Scholar] [CrossRef]
- Xu, H.-S.; Wu, Y.-W.; Xu, S.-F.; Sun, H.-X.; Chen, F.-Y.; Yao, L. Antitumor and immunomodulatory activity of polysaccharides from the roots of Actinidia eriantha. J. Ethnopharmacol. 2009, 125, 310–317. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Coffman, R. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Ann. Rev. Immunol. 1989, 7, 145–173. [Google Scholar] [CrossRef]
- Singh, V.; Mehrotra, S.; Agarwal, S. The paradigm of Th1 and Th2 cytokines. Immunol. Res. 1999, 20, 147–161. [Google Scholar] [CrossRef]
- Constant, S.L.; Bottomly, K. Induction of Th1 and Th2 CD4+ T cell responses: The alternative approaches. Ann. Rev. Immunol. 1997, 15, 297–322. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Sad, S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 1996, 17, 138–146. [Google Scholar] [CrossRef]
- Hoover, S.K.; Barrett, S.K.; Turk, T.M.; Lee, T.-C.; Bear, H.D. Cyclophosphamide and abrogation of tumor-induced suppressor T cell activity. Cancer Immunol. Immunother. 1990, 31, 121–127. [Google Scholar] [CrossRef]
- Monmai, C.; You, S.; Park, W.J. Immune-enhancing effects of anionic macromolecules extracted from Codium fragile on cyclophosphamide-treated mice. PLoS ONE 2019, 14, e0211570. [Google Scholar] [CrossRef]
- Liu, N.; Dong, Z.; Zhu, X.; Xu, H.; Zhao, Z. Characterization and protective effect of Polygonatum sibiricum polysaccharide against cyclophosphamide-induced immunosuppression in Balb/c mice. Int. J. Biol. Macromol. 2018, 107, 796–802. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, F.; Chen, G.; Chen, Y.; Zhang, W.; Mao, G.; Zhao, T.; Zhang, M.; Yang, L.; Wu, X. Purification, characterization and immunomodulatory activity of a novel polysaccharide from Grifola frondosa. Int. J. Biol. Macromol. 2018, 111, 1293–1303. [Google Scholar] [CrossRef]
- Ha, J.-H.; Kwon, M.-C.; Han, J.-G.; Jin, L.; Jeong, H.S.; Choi, G.-P.; Park, U.-Y.; You, S.-G.; Lee, H.-Y. Enhancement of immunomodulatory activities of low molecular weight fucoidan isolated from Hizikia fusiforme. Korean J. Food. Sci. Technol. 2008, 40, 545–550. [Google Scholar]
- Shimizu, J.; Wanda-Funada, U.; Mano, H.; Matahira, Y.; Kawaguchi, M.; Wada, M. Proportion of murine cytotoxic T cells is increased by high molecular-weight fucoidan extracted from Okinawa mozuku (Cladosiphon okamuranus). J. Health Sci. 2005, 51, 394–397. [Google Scholar] [CrossRef]
- Jang, J.Y.; Moon, S.Y.; Joo, H.G. Differential effects of fucoidans with low and high molecular weight on the viability and function of spleen cells. Food Chem. Toxicol. 2014, 68, 234–238. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Filisetti-Cozzi, T.M.; Carpita, N.C. Measurement of uronic acids without interference from neutral sugars. Anal. Biochem. 1991, 197, 157–162. [Google Scholar] [CrossRef]
- Li, W.; Gao, S.; Wang, G.; Cai, L. Cyclophosphamide induces a Fas/FasL-dependent apoptotic cell death in rat thymus. Immunol. J. 2000, 16, 315. [Google Scholar]
- Casteels, K.; Waer, M.; Bouillon, R.; Depovere, J.; Valckx, D.; Laureys, J.; Mathieu, C. 1, 25-Dihydroxyvitamin D3 restores sensitivity to cyclophosphamide-induced apoptosis in non-obese diabetic (NOD) mice and protects against diabetes. Clin. Exp. Immunol. 1998, 112, 181. [Google Scholar] [CrossRef]
- Chung, W.-C.; Chun, S.-h.; Han, J.H.; Song, M.J.; Lee, K.-W. Enhancing the natural killer cell activity and anti-influenza effect of heat-treated Lactobacillus plantarum nF1-fortified yogurt in mice. J. Dairy Sci. 2018, 101, 10675–10684. [Google Scholar]
- Moon, P.-D.; Lee, J.S.; Kim, H.-Y.; Han, N.-R.; Kang, I.; Kim, H.-M.; Jeong, H.-J. Heat-treated Lactobacillus plantarum increases the immune responses through activation of natural killer cells and macrophages on in vivo and in vitro models. J. Med. Microbiol. 2019, 68, 467–474. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]






| Sample | Carbohydrate (%) | Sulfate (%) | Uronic Acid (%) | Monosaccharide Composition | ||
|---|---|---|---|---|---|---|
| Fucose (%) | Galactose (%) | Mannose (%) | ||||
| HMWF 1 | 72.0 ± 3.9 | 30.9 ± 2.7 | 10.9 ± 2.8 | 21.0 ± 2.3 | 23.0 ± 0.8 | 0.9 ± 0.5 |
| LMWF 2 | 73.8 ± 3.3 | 28.8 ± 2.7 | 10.3 ± 2.5 | 21.5 ± 1.5 | 16.7 ± 2.7 | 0.75 ± 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, H.J.; You, D.-J.; Lee, K.-W. Characterization and Immunomodulatory Effects of High Molecular Weight Fucoidan Fraction from the Sporophyll of Undaria pinnatifida in Cyclophosphamide-Induced Immunosuppressed Mice. Mar. Drugs 2019, 17, 447. https://doi.org/10.3390/md17080447
Yoo HJ, You D-J, Lee K-W. Characterization and Immunomodulatory Effects of High Molecular Weight Fucoidan Fraction from the Sporophyll of Undaria pinnatifida in Cyclophosphamide-Induced Immunosuppressed Mice. Marine Drugs. 2019; 17(8):447. https://doi.org/10.3390/md17080447
Chicago/Turabian StyleYoo, Hee Joon, Dong-Ju You, and Kwang-Won Lee. 2019. "Characterization and Immunomodulatory Effects of High Molecular Weight Fucoidan Fraction from the Sporophyll of Undaria pinnatifida in Cyclophosphamide-Induced Immunosuppressed Mice" Marine Drugs 17, no. 8: 447. https://doi.org/10.3390/md17080447
APA StyleYoo, H. J., You, D. -J., & Lee, K. -W. (2019). Characterization and Immunomodulatory Effects of High Molecular Weight Fucoidan Fraction from the Sporophyll of Undaria pinnatifida in Cyclophosphamide-Induced Immunosuppressed Mice. Marine Drugs, 17(8), 447. https://doi.org/10.3390/md17080447