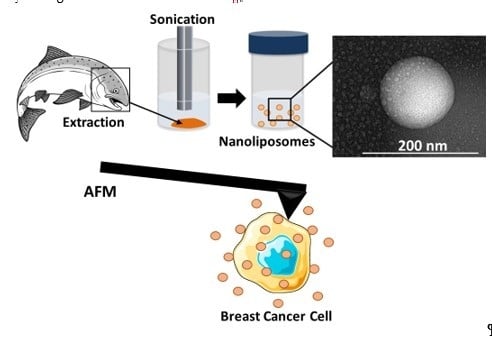
Effects of Bioactive Marine-Derived Liposomes on Two Human Breast Cancer Cell Lines
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Fatty Acid Composition
3.2. Lipid Classes
3.3. Nanoliposome Preparation
3.4. Nanoliposome Size and ζ-Potential Measurements
3.5. Transmission Electron Microscopy (TEM)
3.6. Membrane Fluidity
3.7. Cell Culture
3.8. Cell Viability Assay
3.9. Cell Cycle Analysis
3.10. Nanomechanical Properties by Atomic Force Microscopy (AFM)
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerber, M. Omega-3 fatty acids and cancers: A systematic update review of epidemiological studies. Br. J. Nutr. 2012, 107, S228–S239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.-S.; Hu, X.-J.; Zhao, Y.-M.; Yang, J.; Li, D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: Meta-analysis of data from 21 independent prospective cohort studies. BMJ 2013, 346, f3706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straka, S.; Lester, J.L.; Cole, R.M.; Andridge, R.R.; Puchala, S.; Rose, A.M.; Clinton, S.K.; Belury, M.A.; Yee, L.D. Incorporation of eicosapentaenioic and docosahexaenoic acids into breast adipose tissue of women at high risk of breast cancer: A randomized clinical trial of dietary fish and n-3 fatty acid capsules. Mol. Nutr. Food Res. 2015, 59, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Rani, I.; Bhatnagar, A.; Agnihotri, N. Apoptosis-Mediated Chemoprevention by Different Ratios of Fish Oil in Experimental Colon Carcinogenesis. Cancer Investig. 2016, 34, 220–230. [Google Scholar] [CrossRef]
- Pham, T.-M.; Fujino, Y.; Kubo, T.; Ide, R.; Tokui, N.; Mizoue, T.; Ogimoto, I.; Matsuda, S.; Yoshimura, T. Fish intake and the risk of fatal prostate cancer: Findings from a cohort study in Japan. Public Health Nutr. 2009, 12, 609–613. [Google Scholar] [CrossRef] [Green Version]
- Wen, X.; Reynolds, L.; Mulik, R.S.; Kim, S.Y.; Van Treuren, T.; Nguyen, L.H.; Zhu, H.; Corbin, I.R. Hepatic Arterial Infusion of Low-Density Lipoprotein Docosahexaenoic Acid Nanoparticles Selectively Disrupts Redox Balance in Hepatoma Cells and Reduces Growth of Orthotopic Liver Tumors in Rats. Gastroenterology 2016, 150, 488–498. [Google Scholar] [CrossRef] [Green Version]
- Haqq, J.; Howells, L.M.; Garcea, G.; Dennison, A.R. Targeting pancreatic cancer using a combination of gemcitabine with the omega-3 polyunsaturated fatty acid emulsion, Lipidem. Mol. Nutr. Food Res. 2016, 60, 1437–1447. [Google Scholar] [CrossRef] [Green Version]
- Mozaffarian, D.; Wu, J.H.Y. Omega-3 Fatty Acids and Cardiovascular Disease. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [Green Version]
- Lavie, C.J.; Milani, R.V.; Mehra, M.R.; Ventura, H.O. Omega-3 Polyunsaturated Fatty Acids and Cardiovascular Diseases. J. Am. Coll. Cardiol. 2009, 54, 585–594. [Google Scholar] [CrossRef] [Green Version]
- Calvo, M.J.; Martínez, M.S.; Torres, W.; Chávez-Castillo, M.; Luzardo, E.; Villasmil, N.; Salazar, J.; Velasco, M.; Bermúdez, V. Omega-3 polyunsaturated fatty acids and cardiovascular health: A molecular view into structure and function. Vessel Plus 2017, 1, 116–128. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Luo, C.; Feng, Y.; Yao, X.; Shi, Z.; Liang, F.; Kang, J.X.; Wan, J.-B.; Pei, Z.; Su, H. Omega-3 polyunsaturated fatty acids promote amyloid-β clearance from the brain through mediating the function of the glymphatic system. FASEB J. 2017, 31, 282–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denis, I.; Potier, B.; Heberden, C.; Vancassel, S. Omega-3 polyunsaturated fatty acids and brain aging. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wachtel, N.; Rohwer, N.; Pietzner, A.; Loew, A.; Weylandt, K.H. Omega-3 fatty acid supplementation—A possible dietary adjunct to enhance immune checkpoint inhibition therapy in cancer? JCB 2019, 4, 83–88. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Omega-3 fatty acids and inflammation. Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corsetto, P.; Colombo, I.; Kopecka, J.; Rizzo, A.; Riganti, C. ω-3 Long Chain Polyunsaturated Fatty Acids as Sensitizing Agents and Multidrug Resistance Revertants in Cancer Therapy. IJMS 2017, 18, 2770. [Google Scholar] [CrossRef] [Green Version]
- Allam-Ndoul, B.; Guénard, F.; Barbier, O.; Vohl, M.-C. A Study of the Differential Effects of Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA) on Gene Expression Profiles of Stimulated Thp-1 Macrophages. Nutrients 2017, 9, 424. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, S.; Wu, Y.; He, Z.; Chen, Y. Omega-3 free fatty acids attenuate insulin-promoted breast cancer cell proliferation. Nutr. Res. 2017, 42, 43–50. [Google Scholar] [CrossRef]
- Bianchini, F.; Giannoni, E.; Serni, S.; Chiarugi, P.; Calorini, L. 22:6 n-3 DHA inhibits differentiation of prostate fibroblasts into myofibroblasts and tumorigenesis. Br. J. Nutr. 2012, 108, 2129–2137. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Jia, X.; Hou, L.; Liu, X.; Gao, Q. Involvement of apoptotic pathways in docosahexaenoic acid-induced benefit in prostate cancer: Pathway-focused gene expression analysis using RT2 Profile PCR Array System. Lipids Health Dis. 2017, 16, 59. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.; Elkhoury, K.; Kahn, C.J.F.; Arab-Tehrany, E.; Linder, M. Preparation, Characterization, and Release Kinetics of Chitosan-Coated Nanoliposomes Encapsulating Curcumin in Simulated Environments. Molecules 2019, 24, 2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.M.; Veigas, J.M.; Williams, P.J.; Fernandes, G. DHA is a more potent inhibitor of breast cancer metastasis to bone and related osteolysis than EPA. Breast Cancer Res. Treat. 2013, 141, 341–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.L.; Nguyen, T.H.; Nguyen, D.H. Development and In Vitro Evaluation of Liposomes Using Soy Lecithin to Encapsulate Paclitaxel. Int. J. Biomater. 2017, 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Aslan, B.; Ozpolat, B.; Sood, A.K.; Lopez-Berestein, G. Nanotechnology in cancer therapy. J. Drug Target. 2013, 21, 904–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, D.H.; Lee, J.S.; Bae, J.W.; Choi, J.H.; Lee, Y.; Son, J.Y.; Park, K.D. Targeted doxorubicin nanotherapy strongly suppressing growth of multidrug resistant tumor in mice. Int. J. Pharm. 2015, 495, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Chang, L.; Kuang, T.; Hu, J. PEG/heparin-decorated lipid–polymer hybrid nanoparticles for long-circulating drug delivery. RSC Adv. 2016, 6, 23279–23287. [Google Scholar] [CrossRef]
- Elkhoury, K.; Russell, C.S.; Sanchez-Gonzalez, L.; Mostafavi, A.; Williams, T.J.; Kahn, C.; Peppas, N.A.; Arab-Tehrany, E.; Tamayol, A. Soft-Nanoparticle Functionalization of Natural Hydrogels for Tissue Engineering Applications. Adv. Healthc. Mater. 2019, 8, 1900506. [Google Scholar] [CrossRef]
- Hasan, M.; Belhaj, N.; Benachour, H.; Barberi-Heyob, M.; Kahn, C.J.F.; Jabbari, E.; Linder, M.; Arab-Tehrany, E. Liposome encapsulation of curcumin: Physico-chemical characterizations and effects on MCF7 cancer cell proliferation. Int. J. Pharm. 2014, 461, 519–528. [Google Scholar] [CrossRef]
- Hasan, M.; Latifi, S.; Kahn, C.; Tamayol, A.; Habibey, R.; Passeri, E.; Linder, M.; Arab-Tehrany, E. The Positive Role of Curcumin-Loaded Salmon Nanoliposomes on the Culture of Primary Cortical Neurons. Mar. Drugs 2018, 16, 218. [Google Scholar] [CrossRef] [Green Version]
- Latifi, S.; Tamayol, A.; Habibey, R.; Sabzevari, R.; Kahn, C.; Geny, D.; Eftekharpour, E.; Annabi, N.; Blau, A.; Linder, M.; et al. Natural lecithin promotes neural network complexity and activity. Sci. Rep. 2016, 6, 25777. [Google Scholar] [CrossRef]
- Burns, C.P.; Spector, A.A. Effects of Lipids on Cancer Therapy. Nutr. Rev. 2009, 48, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, T.; Geng, Y.; Peyton, S.; Mercurio, A.M.; Rotello, V.M. Biochemical and biomechanical drivers of cancer cell metastasis, drug response and nanomedicine. Drug Discov. Today 2016, 21, 1489–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manca, M.L.; Castangia, I.; Matricardi, P.; Lampis, S.; Fernàndez-Busquets, X.; Fadda, A.M.; Manconi, M. Molecular arrangements and interconnected bilayer formation induced by alcohol or polyalcohol in phospholipid vesicles. Colloids Surf. B Biointerf. 2014, 117, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Paolino, D.; Fresta, M.; Sinha, D.; Ferrari, M. Drug delivery systems. In Encyclopedia of Medical Devices and Instrumentation, 2nd ed.; Webester, J.G., Ed.; John Wiley and Sons: Hoboken, NJ, USA, 2006; pp. 437–495. [Google Scholar]
- Mady, M.M.; Darwish, M.M.; Khalil, S.; Khalil, W.M. Biophysical studies on chitosan-coated liposomes. Eur. Biophys. J. Biophys. Lett. 2009, 38, 1127–1133. [Google Scholar] [CrossRef]
- Maherani, B.; Arab-Tehrany, E.; Kheirolomoom, A.; Cleymand, F.; Linder, M. Influence of lipid composition on physicochemical properties of nanoliposomes encapsulating natural dipeptide antioxidant l-carnosine. Food Chem. 2012, 134, 632–640. [Google Scholar] [CrossRef]
- Leekumjorn, S.; Cho, H.J.; Wu, Y.; Wright, N.T.; Sum, A.K.; Chan, C. The role of fatty acid unsaturation in minimizing biophysical changes on the structure and local effects of bilayer membranes. Biochim. Biophys. Acta Biomembr. 2009, 1788, 1508–1516. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.; Ben Messaoud, G.; Michaux, F.; Tamayol, A.; Kahn, C.J.F.; Belhaj, N.; Linder, M.; Arab-Tehrany, E. Chitosan-coated liposomes encapsulating curcumin: Study of lipid-polysaccharide interactions and nanovesicle behavior. RSC Adv. 2016, 6, 45290–45304. [Google Scholar] [CrossRef]
- Stubbs, C.D.; Smith, A.D. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim. Biophys. Acta Rev. Biomembr. 1984, 779, 89–137. [Google Scholar] [CrossRef]
- Mouritsen, O.G.; Zuckermann, M.J. Softening of lipid bilayers. Eur. Biophys. J. 1985, 12, 75–86. [Google Scholar] [CrossRef]
- Chekashkina, K.; Kuzmin, P.; Bashkirov, P.; Frolov, V. Lipids as Regulators of Effective Membrane Rigidity. Biophys. J. 2014, 106, 288. [Google Scholar] [CrossRef] [Green Version]
- Bouarab, L.; Maherani, B.; Kheirolomoom, A.; Hasan, M.; Aliakbarian, B.; Linder, M.; Arab-Tehrany, E. Influence of lecithin-lipid composition on physico-chemical properties of nanoliposomes loaded with a hydrophobic molecule. Colloids Surf. B Biointerf. 2014, 115, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Beblik, G.; Servuss, R.-M.; Helfrich, W. Bilayer bending rigidity of some synthetic lecithins. J. Phys. France 1985, 46, 1773–1778. [Google Scholar] [CrossRef]
- Sarfraz, M.; Afzal, A.; Yang, T.; Gai, Y.; Raza, S.; Khan, M.; Cheng, Y.; Ma, X.; Xiang, G. Development of Dual Drug Loaded Nanosized Liposomal Formulation by A Reengineered Ethanolic Injection Method and Its Pre-Clinical Pharmacokinetic Studies. Pharmaceutics 2018, 10, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bordeleau, F.; Mason, B.N.; Lollis, E.M.; Mazzola, M.; Zanotelli, M.R.; Somasegar, S.; Califano, J.P.; Montague, C.; LaValley, D.J.; Huynh, J.; et al. Matrix stiffening promotes a tumor vasculature phenotype. Proc. Natl. Acad. Sci. USA 2017, 114, 492–497. [Google Scholar] [CrossRef] [Green Version]
- Marangon, I.; Silva, A.A.K.; Guilbert, T.; Kolosnjaj-Tabi, J.; Marchiol, C.; Natkhunarajah, S.; Chamming’s, F.; Ménard-Moyon, C.; Bianco, A.; Gennisson, J.-L.; et al. Tumor Stiffening, a Key Determinant of Tumor Progression, is Reversed by Nanomaterial-Induced Photothermal Therapy. Theranostics 2017, 7, 329–343. [Google Scholar] [CrossRef]
- Spill, F.; Reynolds, D.S.; Kamm, R.D.; Zaman, M.H. Impact of the physical microenvironment on tumor progression and metastasis. Curr. Opin. Biotechnol. 2016, 40, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Emon, B.; Bauer, J.; Jain, Y.; Jung, B.; Saif, T. Biophysics of Tumor Microenvironment and Cancer Metastasis—A Mini Review. Comput. Struct. Biotechnol. J. 2018, 16, 279–287. [Google Scholar] [CrossRef]
- Linder, M.; Matouba, E.; Fanni, J.; Parmentier, M. Enrichment of salmon oil with n-3 PUFA by lipolysis, filtration and enzymatic re-esterification. Eur. J. Lipid Sci. Technol. 2002, 104, 455–462. [Google Scholar] [CrossRef]
- Ackman, R.G. Remarks on official methods employing boron trifluoride in the preparation of methyl esters of the fatty acids of fish oils. J. Am. Oil Chem. Soc. 1998, 75, 541–545. [Google Scholar] [CrossRef]
- Maherani, B.; Arab-Tehrany, E.; Kheirolomoom, A.; Reshetov, V.; Stebe, M.J.; Linder, M. Optimization and characterization of liposome formulation by mixture design. Analyst 2012, 137, 773–786. [Google Scholar] [CrossRef]
- Gavara, N.; Chadwick, R.S. Determination of the elastic moduli of thin samples and adherent cells using conical atomic force microscope tips. Nat. Nanotechnol. 2012, 7, 733–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polyakov, P.; Soussen, C.; Duan, J.; Duval, J.F.L.; Brie, D.; Francius, G. Automated Force Volume Image Processing for Biological Samples. PLoS ONE 2011, 6, e18887. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Fatty Acids | Lecithin (%) | SD |
|---|---|---|
| C14:0 | 1.23 | 0.01 |
| C16:0 | 12.09 | 0.03 |
| C18:0 | 4.11 | 0.04 |
| C21:0 | 1.77 | 0.01 |
| C23:0 | 1.44 | 0.01 |
| SFA | 20.64 | - |
| C16:1 | 1.21 | 0.06 |
| C17:1 | 1.3 | 0.08 |
| C18:1n9 | 20.2 | 0.01 |
| MUFA | 22.71 | - |
| C18:2n6 | 5.79 | 0.01 |
| C18:3n3 | 4.67 | 0.02 |
| C20:4n6 | 2.26 | 0.08 |
| C20:5n3 (EPA) | 9.54 | 0.06 |
| C22:4n6 | 1.24 | 0.02 |
| C22:5n3 | 3.03 | 0.06 |
| C22:6n3 (DHA) | 30.12 | 0.29 |
| PUFA | 56.65 | - |
| MCF7 Cells | # Datum | MDA-MB-231 | # Datum | |
|---|---|---|---|---|
| No treatment | 5.39 ± 3.51 kPa | 5745 | 5.06 ± 3.27 kPa | 6258 |
| After 48 h | 3.06 ± 2.32 kPa | 10,203 | 2.94 ± 2.21 kPa | 5867 |
| After 96 h | 2.74 ± 2.52 kPa | 8505 | 2.72 ± 2.41 kPa | 9318 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Elkhoury, K.; Barbieux, C.; Linder, M.; Grandemange, S.; Tamayol, A.; Francius, G.; Arab-Tehrany, E. Effects of Bioactive Marine-Derived Liposomes on Two Human Breast Cancer Cell Lines. Mar. Drugs 2020, 18, 211. https://doi.org/10.3390/md18040211
Li J, Elkhoury K, Barbieux C, Linder M, Grandemange S, Tamayol A, Francius G, Arab-Tehrany E. Effects of Bioactive Marine-Derived Liposomes on Two Human Breast Cancer Cell Lines. Marine Drugs. 2020; 18(4):211. https://doi.org/10.3390/md18040211
Chicago/Turabian StyleLi, Jie, Kamil Elkhoury, Claire Barbieux, Michel Linder, Stéphanie Grandemange, Ali Tamayol, Grégory Francius, and Elmira Arab-Tehrany. 2020. "Effects of Bioactive Marine-Derived Liposomes on Two Human Breast Cancer Cell Lines" Marine Drugs 18, no. 4: 211. https://doi.org/10.3390/md18040211
APA StyleLi, J., Elkhoury, K., Barbieux, C., Linder, M., Grandemange, S., Tamayol, A., Francius, G., & Arab-Tehrany, E. (2020). Effects of Bioactive Marine-Derived Liposomes on Two Human Breast Cancer Cell Lines. Marine Drugs, 18(4), 211. https://doi.org/10.3390/md18040211