Sea Urchin Pigments: Echinochrome A and Its Potential Implication in the Cytokine Storm Syndrome
Abstract
:1. Introduction
2. Echinochrome A and Its Pharmacological Applications
3. Echinochrome A and Redox Imbalance
4. Echinochrome A and Immune System Response
5. Cytokine Storm Syndrome
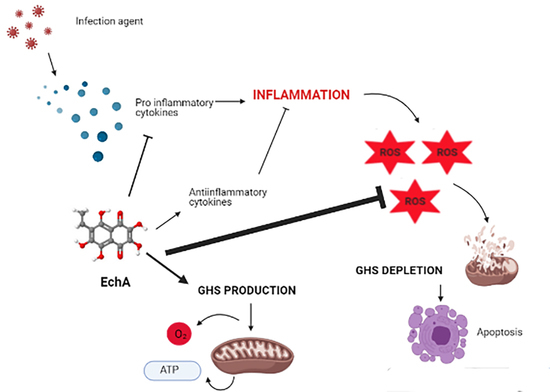
6. Echinochrome A and Cytokine Storm Syndrome
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jeong, S.H.; Kim, H.K.; Song, I.-S.; Lee, S.J.; Ko, K.S.; Rhee, B.D.; Kim, N.; Mishchenko, N.P.; Fedoryev, S.A.; Stonik, V.A.; et al. Echinochrome A Protects Mitochondrial Function in Cardiomyocytes against Cardiotoxic Drugs. Mar. Drugs 2014, 12, 2922–2936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayed, D.A.; Soliman, A.M.; Fahmy, S.R. Echinochrome pigment as novel therapeutic agent against experimentally-induced gastric ulcer in rats. Biomed. Pharmacother. 2018, 107, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.M.; Mohamed, A.S.; Marie, M.-A.S. Echinochrome pigment attenuates diabetic nephropathy in the models of type 1 and type 2 diabetes. Diabetes Mellit. 2016, 19, 464–470. [Google Scholar] [CrossRef] [Green Version]
- Yoon, C.S.; Kim, H.K.; Mishchenko, N.P.; Vasileva, E.A.; Fedoreyev, S.A.; Shestak, O.P.; Balaneva, N.N.; Novikov, V.L.; Stonik, V.A.; Han, J. The protective effects of echinochrome A structural analog against oxidative stress and doxorubicin in AC16 cardiomyocytes. Mol. Cell. Toxicol. 2019, 15, 407–414. [Google Scholar] [CrossRef]
- Shikov, A.N.; Pozharitskaya, O.N.; Krishtopina, A.S.; Makarov, V.G. Naphthoquinone pigments from sea urchins: Chemistry and pharmacology. Phytochem. Rev. 2018, 17, 509–534. [Google Scholar] [CrossRef]
- Brasseur, L.; Hennebert, E.; Fievez, L.; Caulier, G.; Bureau, F.; Tafforeau, L.; Flammang, P.; Gerbaux, P.; Eeckhaut, I. The Roles of Spinochromes in Four Shallow Water Tropical Sea Urchins and Their Potential as Bioactive Pharmacological Agents. Mar. Drugs 2017, 15, 179. [Google Scholar] [CrossRef] [Green Version]
- Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A. Diversity of Polyhydroxynaphthoquinone Pigments in North Pacific Sea Urchins. Chem. Biodivers. 2017, 14, e1700182. [Google Scholar] [CrossRef]
- Nishibori, K. Isolation of Echinochrome A from the Spines of the Sea Urchin, Diadema setosum (Leske). Nature 1959, 184, 1234. [Google Scholar] [CrossRef]
- Martínez, M.J.A.; Benito, P.B. Biological Activity of Quinones. In Studies in Natural Products Chemistry; Elsevier BV: Amsterdam, The Netherlands, 2005; Volume 30, pp. 303–366. [Google Scholar]
- Zhou, D.-Y.; Qin, L.; Zhu, B.-W.; Wang, X.-D.; Tan, H.; Yang, J.-F.; Li, D.-M.; Dong, X.-P.; Wu, H.-T.; Sun, L.-M.; et al. Extraction and antioxidant property of polyhydroxylated naphthoquinone pigments from spines of purple sea urchin Strongylocentrotus nudus. Food Chem. 2011, 129, 1591–1597. [Google Scholar] [CrossRef]
- Shikov, A.N.; Flisyuk, E.V.; Obluchinskaya, E.D.; Pozharitskaya, O.N. Pharmacokinetics of Marine-Derived Drugs. Mar. Drugs 2020, 18, 557. [Google Scholar] [CrossRef]
- Artyukov, A.A.; Zelepuga, E.A.; Bogdanovich, L.N.; Lupach, N.M.; Novikov, V.L.; Rutckova, T.A.; Kozlovskaya, E.P. Marine Polyhydroxynaphthoquinone, Echinochrome A: Prevention of Atherosclerotic Inflammation and Probable Molecular Targets. J. Clin. Med. 2020, 9, 1494. [Google Scholar] [CrossRef]
- Pozharitskaya, O.; Shikov, A.; Makarova, M.; Ivanova, S.; Kosman, V.; Makarov, V.G.; Bazgier, V.; Berka, K.; Otyepka, M.; Ulrichová, J. Antiallergic Effects of Pigments Isolated from Green Sea Urchin (Strongylocentrotus droebachiensis) Shells. Planta Med. 2013, 79, 1698–1704. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Fujiwara, A.; Ninomiya, M.; Maeda, T.; Ando, M.; Tsukamasa, Y.; Koketsu, M. Inhibitory Effects of Echinochrome A, Isolated from Shell of the Sea Urchin Anthocidaris crassispina, on Antigen-Stimulated Degranulation in Rat Basophilic Leukemia RBL-2H3 Cells through Suppression of Lyn Activation. Nat. Prod. Commun. 2016, 11, 1303–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lebed’Ko, O.A.; Ryzhavskii, B.Y.; Demidova, O.V. Effect of Antioxidant Echinochrome A on Bleomycin-Induced Pulmonary Fibrosis. Bull. Exp. Biol. Med. 2015, 159, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-J.; Seo, Y.; Ahn, J.-S.; Shin, Y.Y.; Yang, J.W.; Kim, H.K.; Han, J.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; et al. Echinochrome A Reduces Colitis in Mice and Induces In Vitro Generation of Regulatory Immune Cells. Mar. Drugs 2019, 17, 622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishchenko, N.P.; Fedoreev, S.A.; Bagirova, V.L. Histochrome: A New Original Domestic Drug. Pharm. Chem. J. 2003, 37, 48–52. [Google Scholar] [CrossRef]
- Lee, S.R.; Pronto, J.R.D.; Sarankhuu, B.-E.; Ko, K.S.; Rhee, B.D.; Kim, N.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Han, J. Acetylcholinesterase Inhibitory Activity of Pigment Echinochrome A from Sea Urchin Scaphechinus mirabilis. Mar. Drugs 2014, 12, 3560–3573. [Google Scholar] [CrossRef] [Green Version]
- Kareva, E.N.; Tikhonov, D.A.; Mishchenko, N.P.; Fedoreev, S.A.; Shimanovskii, N.L. Effects of Histochrome on P53 Expression in Mouse Red Bone Marrow Cells in a Model of Chronic Stress. Pharm. Chem. J. 2014, 48, 149–152. [Google Scholar] [CrossRef]
- Lennikov, A.; Kitaichi, N.; Noda, K.; Mizuuchi, K.; Ando, R.; Dong, Z.; Fukuhara, J.; Kinoshita, S.; Namba, K.; Ohno, S.; et al. Amelioration of endotoxin-induced uveitis treated with the sea urchin pigment echinochrome in rats. Mol. Vis. 2014, 20, 171–177. [Google Scholar]
- Artyukov, A.A.; Popov, A.M.; Tsybulsky, A.V.; Krivoshapko, O.N.; Polyakova, N.V. Pharmacological activity of echinochrome a alone and in the biologically active additive Timarin. Biochem. Suppl. Ser. B Biomed. Chem. 2013, 7, 237–242. [Google Scholar] [CrossRef]
- Mohamed, A.S.; Soliman, A.M.; Marie, M.A.S. Mechanisms of echinochrome potency in modulating diabetic complications in liver. Life Sci. 2016, 151, 41–49. [Google Scholar] [CrossRef]
- Soliman, A.M.; Mohamed, A.S.; Assem, M.; Marie, S. Effect of echinochrome on body weight, musculoskeletal system and lipid profile of male diabetic rats. Austin J. Endocrinol. Diabetes 2016, 3, 1045. [Google Scholar]
- Seo, D.Y.; McGregor, R.A.; Noh, S.J.; Choi, S.J.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Han, J. Echinochrome A Improves Exercise Capacity during Short-Term Endurance Training in Rats. Mar. Drugs 2015, 13, 5722–5731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekimova, I.V.; Plaksina, D.V.; Pastukhov, Y.F.; Lapshina, K.V.; Lazarev, V.F.; Mikhaylova, E.R.; Polonik, S.G.; Pani, B.; Margulis, B.A.; Guzhova, I.V.; et al. New HSF1 inducer as a therapeutic agent in a rodent model of Parkinson’s disease. Exp. Neurol. 2018, 306, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.R.; A Sayed, D.; Soliman, A.M.; Almortada, N.Y.; Aal, W.E.A.-E. Protective effect of Echinochrome against intrahepatic cholestasis induced by alpha-naphthylisothiocyanate in rats. Braz. J. Biol. 2020, 80, 102–111. [Google Scholar] [CrossRef]
- Kim, H.K.; Cho, S.W.; Heo, H.J.; Jeong, S.H.; Kim, M.; Ko, K.S.; Rhee, B.D.; Mishchenko, N.P.; Vasileva, E.A.; Fedoreyev, S.A.; et al. A Novel Atypical PKC-Iota Inhibitor, Echinochrome A, Enhances Cardiomyocyte Differentiation from Mouse Embryonic Stem Cells. Mar. Drugs 2018, 16, 192. [Google Scholar] [CrossRef] [Green Version]
- Tsybulsky, A.V.; Popov, A.M.; Klimovich, A.A.; Artyukov, A.A.; Kostetsky, E.Y.; Veselova, M.D. Comparative study of echinochrome a, oxygenated carotenoids, ginsenoside Rh2, luteolin disulfate and metformin as a mean to potentiate antitumor effect of doxorubicin. Med. Immunol. 2018, 20, 179–192. [Google Scholar] [CrossRef] [Green Version]
- Yoon, C.S.; Kim, H.K.; Mishchenko, N.P.; Vasileva, E.A.; Fedoreyev, S.A.; Stonik, V.A.; Han, J. Spinochrome D Attenuates Doxorubicin-Induced Cardiomyocyte Death via Improving Glutathione Metabolism and Attenuating Oxidative Stress. Mar. Drugs 2018, 17, 2. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.S.; Sadek, S.A.; Hassanein, S.S.; Soliman, A.M. Hepatoprotective Effect of Echinochrome Pigment in Septic Rats. J. Surg. Res. 2019, 234, 317–324. [Google Scholar] [CrossRef]
- Fahmy, S.R.; Zaki, N.I.; Eid, S.Z.; Mohamed, A.S.; Hassanein, S.S. Effectiveness of Echinochrome on HFD-Induced Hyperlipidemia in Rats. Nat. Prod. Bioprospect. 2019, 9, 337–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.M.; Kim, J.H.; Shin, S.-C.; Park, G.C.; Kim, H.S.; Kim, K.; Kim, H.K.; Han, J.; Mishchenko, N.P.; Vasileva, E.A.; et al. The Protective Effect of Echinochrome A on Extracellular Matrix of Vocal Folds in Ovariectomized Rats. Mar. Drugs 2020, 18, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, A.S. Echinochrome Exhibits Antitumor Activity against Ehrlich Ascites Carcinoma in Swiss Albino Mice. Nutr. Cancer 2021, 73, 124–132. [Google Scholar] [CrossRef]
- Talalaeva, O.; Mishchenko, N.; Bryukhanov, V.; Zverev, Y.; Fedoreyev, S.A.; Lampatov, V.; Zharikov, A. The influence of histochrome on exudative and proliferative phases of the experimental inflammation. Sib. Sci. Med. J. 2012, 32, 28–31. [Google Scholar]
- Ivanov, A.V.; Valuev-Elliston, V.T.; Ivanova, O.N.; Kochetkov, S.N.; Starodubova, E.S.; Bartosch, B.; Isaguliants, M.G. Oxida-tive Stress during HIV Infection: Mechanisms and Consequences. Oxid. Med. Cell. Longev. 2016, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Akaike, T.; Suga, M.; Maeda, H. Free radicals in viral pathogenesis: Molecular mechanisms involving superoxide and NO. Proc. Soc. Exp. Boil. Med. 1998, 217, 64–73. [Google Scholar] [CrossRef]
- Ramm, G.A.; Ruddell, R.G. Hepatotoxicity of Iron Overload: Mechanisms of Iron-Induced Hepatic Fibrogenesis. Semin. Liver Dis. 2005, 25, 433–449. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Motohashi, H.; Yamamoto, M. Nrf2–Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Gough, N.R. Focus Issue: The Long and Short of Redox Signaling. Sci. Signal. 2009, 2, eg12. [Google Scholar] [CrossRef]
- Günther, T.M.F.; Grinevicius, V.M.; Pedrosa, R.C. Active Learning Using Protein Data Bank (PDB) Biochemical Data by Undergraduate Students of Nutrition Course at UFSC. Revista de Ensino de Bioquímica 2018, 16, 11. [Google Scholar] [CrossRef]
- Irrcher, I.; Ljubicic, V.; Hood, D.A. Interactions between ROS and AMP kinase activity in the regulation of PGC-1α transcription in skeletal muscle cells. Am. J. Physiol. Physiol. 2009, 296, C116–C123. [Google Scholar] [CrossRef] [Green Version]
- Sung, D.J.; So, W.Y.; Ryu, H.Y.; An, H.S.; Cha, K.S. Induction of vasodilation by hydro-gen peroxide and its application in exercise science. Biol. Sport 2012, 29, 87–92. [Google Scholar] [CrossRef]
- Camini, F.C.; da Silva Caetano, C.C.; Almeida, L.T.; de Brito Magalhães, C.L. Implications of oxidative stress on viral path-ogenesis. Arch. Virol. 2017, 162, 907–917. [Google Scholar] [CrossRef]
- Tsybulsky, A.V.; Popov, A.M.; Artyukov, A.A.; Mazeika, A.N.; Kostetsky, E.A.; Sartina, N.M.; Krivoshapko, O.N. Enhancing the immunogenic activity of influvac vaccine in the use of adjuvant TI complexes modified by echinochrome A. Vopr. Virusol. 2012, 57, 23–27. [Google Scholar]
- Busquets-Cortés, C.; Capó, X.; Argelich, E.; Ferrer, M.D.; Mateos, D.; Bouzas, C.; Abbate, M.; Tur, J.A.; Sureda, A.; Pons, A. Effects of Millimolar Steady-State Hydrogen Peroxide Exposure on Inflammatory and Redox Gene Expression in Immune Cells from Humans with Metabolic Syndrome. Nutrients 2018, 10, 1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ball, J.A.; Vlisidou, I.; Blunt, M.D.; Wood, W.; Ward, S.G. Hydrogen Peroxide Triggers a Dual Signaling Axis To Selectively Suppress Activated Human T Lymphocyte Migration. J. Immunol. 2017, 198, 3679–3689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waring, P.; Müllbacher, A. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol. Cell Biol. 1999, 77, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; McAuley, D.F.; Brown, M.; Sanchez, E.; Tattersall, R.S.; Manson, J.J. COVID-19: Consider cytokine storm syn-dromes and immunosuppression. Lancet 2020, 395, 1033–1034. [Google Scholar] [CrossRef]
- Henderson, L.A.; Canna, S.W.; Schulert, G.S.; Volpi, S.; Lee, P.Y.; Kernan, K.F.; Caricchio, R.; Mahmud, S.; Hazen, M.M.; Halyabar, O.; et al. On the Alert for Cytokine Storm: Immunopathology in COVID -19. Arthritis Rheumatol. 2020, 72, 1059–1063. [Google Scholar] [CrossRef] [Green Version]
- Ruscitti, P.; Berardicurti, O.; Iagnocco, A.; Giacomelli, R. Cytokine storm syndrome in severe COVID-19. Autoimmun. Rev. 2020, 19, 102562. [Google Scholar] [CrossRef] [PubMed]
- Konig, M.F.; Powell, M.A.; Staedtke, V.; Bai, R.-Y.; Thomas, D.L.; Fischer, N.M.; Huq, S.; Khalafallah, A.M.; Koenecke, A.; Xiong, R.; et al. Preventing cytokine storm syndrome in COVID-19 using α-1 adrenergic receptor antagonists. J. Clin. Investig. 2020, 130, 3345–3347. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, G.; Wang, B.; Liu, B. Cytokine storm syndrome in coronavirus disease 2019: A narrative review. J. Intern. Med. 2021, 289, 147–161. [Google Scholar] [CrossRef]
- Sinha, P.; Matthay, M.A.; Calfee, C.S. Is a “cytokine Storm” Relevant to COVID-19? JAMA Intern. Med. 2020, 180, 1152–1154. [Google Scholar] [CrossRef]
- Canna, S.W.; Behrens, E.M. Making Sense of the Cytokine Storm: A Conceptual Framework for Understanding, Diagnosing, and Treating Hemophagocytic Syndromes. Pediatr. Clin. N. Am. 2012, 59, 329–344. [Google Scholar] [CrossRef] [Green Version]
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the Eye of the Cytokine Storm. Microbiol. Mol. Biol. Rev. 2012, 76, 16–32. [Google Scholar] [CrossRef] [Green Version]
- Behrens, E.M.; Koretzky, G.A. Review: Cytokine Storm Syndrome: Looking Toward the Precision Medicine Era. Arthritis Rheumatol. 2017, 69, 1135–1143. [Google Scholar] [CrossRef] [Green Version]
- Chousterman, B.G.; Swirski, F.K.; Weber, G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017, 39, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.; Iqbal, M.; Chavez, J.C.; Kharfan-Dabaja, M.A. Cytokine Release Syndrome: Current Perspectives. ImmunoTargets Ther. 2019, 8, 43–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.-W.; Peng, T.-T.; Hsu, H.-Y.; Lee, Y.-Z.; Wu, S.-H.; Lin, W.-H.; Ke, Y.-Y.; Hsu, T.-A.; Yeh, T.-K.; Huang, W.-Z.; et al. Repurposing old drugs as antiviral agents for coronaviruses. Biomed. J. 2020, 43, 368–374. [Google Scholar] [CrossRef]
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef]
- Jesenak, M.; Brndiarova, M.; Urbancikova, I.; Rennerova, Z.; Vojtkova, J.; Bobcakova, A.; Ostro, R.; Banovcin, P. Immune Parameters and COVID-19 Infection–Associations With Clinical Severity and Disease Prognosis. Front. Cell. Infect. Microbiol. 2020, 10, 364. [Google Scholar] [CrossRef]
- Moore, B.J.B.; June, C.H. Cytokine release syndrome in severe COVID-19. Science 2020, 368, 473–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crayne, C.B.; Albeituni, S.; Nichols, K.E.; Cron, R.Q. The Immunology of Macrophage Activation Syndrome. Front. Immunol. 2019, 10, 119. [Google Scholar] [CrossRef] [Green Version]
- Lukan, N. “Cytokine storm”, not only in COVID-19 patients. Mini-review. Immunol. Lett. 2020, 228, 38–44. [Google Scholar] [CrossRef]
- Dinarello, C.A. Immunological and Inflammatory Functions of the Interleukin-1 Family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef] [PubMed]
- Guloyan, V.; Oganesian, B.; Baghdasaryan, N.; Yeh, C.; Singh, M.; Guilford, F.; Ting, Y.-S.; Venketaraman, V. Glutathione Supplementation as an Adjunctive Therapy in COVID-19. Antioxidants 2020, 9, 914. [Google Scholar] [CrossRef]
- Arnalich, F.M.; Hernanz, A.; López-Maderuelo, D.; De La Fuente, M.; Andrés-Mateos, E.; Fernández-Capitán, C.; Montiel, C. Intracellular glutathione deficiency is associated with enhanced nuclear factor-κB activation in older noninsulin dependent diabetic patients. Free Radic. Res. 2001, 35, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Samiec, P.S.; Drews-Botsch, C.; Flagg, E.W.; Kurtz, J.C.; Sternberg, P.; Reed, R.L.; Jones, D.P. Glutathione in Human Plasma: Decline in Association with Aging, Age-Related Macular Degeneration, and Diabetes. Free Radic. Biol. Med. 1998, 24, 699–704. [Google Scholar] [CrossRef]
- McGrowdera, D.; Ragoobirsingh, D.; Brown, P. Modulation of glucose uptake in adipose tissue by nitric oxide-generating compounds. J. Biosci. 2006, 31, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.B.; Guarino, M.P.; Macedo, M.P. Understanding the in-vivo relevance of S-nitrosothiols in insulin action. Can. J. Physiol. Pharmacol. 2012, 90, 887–894. [Google Scholar] [CrossRef]
- Durand, M.; Troyanov, Y.; Laflamme, P.; Gregoire, G. Macrophage Activation Syndrome Treated with Anakinra. J. Rheumatol. 2010, 37, 879–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miettunen, P.M.; Narendran, A.; Jayanthan, A.; Behrens, E.M.; Cron, R.Q. Successful treatment of severe paediatric rheumatic disease-associated macrophage activation syndrome with interleukin-1 inhibition following conventional immunosup-pressive therapy: Case series with 12 patients. Rheumatology 2011, 50, 417–419. [Google Scholar] [CrossRef] [Green Version]
- Hay, K.A. Cytokine release syndrome and neurotoxicity after CD19 chimeric antigen receptor-modified (CAR-) T cell therapy. Br. J. Haematol. 2018, 183, 364–374. [Google Scholar] [CrossRef] [Green Version]
- Tsai, A.; Diawara, O.; Nahass, R.G.; Brunetti, L. Impact of tocilizumab administration on mortality in severe COVID-19. Sci. Rep. 2020, 10, 1–7. [Google Scholar] [CrossRef]
- Sordillo, P.P.; Helson, L. Curcumin suppression of cytokine release and cytokine storm. A potential therapy for patients with Ebola and other severe viral infections. In Vivo 2015, 29, 1–4. [Google Scholar] [PubMed]
- Qin, M.; Cao, Z.; Wen, J.; Yu, Q.; Liu, C.; Wang, F.; Zhang, J.; Yang, F.; Li, Y.; Fishbein, G.; et al. An Antioxidant Enzyme Therapeutic for COVID-19. Adv. Mater. 2020, 32, 2004901. [Google Scholar] [CrossRef]
- Geiler, J.; Michaelis, M.; Naczk, P.; Leutz, A.; Langer, K.; Doerr, H.-W.; Cinatl, J. N-acetyl-l-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza A virus. Biochem. Pharmacol. 2010, 79, 413–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehran, R.; Caixeta, A. N-acetilcisteína en la prevención de la nefropatía inducida por contraste. Administrar o no admin-istrar: Ésta es la cuestión. Rev. Esp. Cardiol. 2010, 63, 9–11. [Google Scholar] [CrossRef]




| Spinochrome | Effect | Experimental model | Reference |
|---|---|---|---|
| Spinochrome D, E | Antiallergic effect | Guinea pigs | [13] |
| Echinochrome A | Antigen-stimulated degranulation in cellular systems | RBL-2H3 cells | [14] |
| Echinochrome A | Antioxidant on bleomycin-induced pulmonary fibrosis | Rats | [15] |
| Echinochrome A | Inflammatory bowel disease and correcting immune system imbalance | Mouse | [16] |
| Echinochrome A | Cardioprotective activity | Human | [17] |
| Echinochrome A | Acetylcholinesterase inhibition | H9c2 and A7r5 cells | [18] |
| Echinochrome A | Antistress effect | Bone marrow cells in SHK mice | [19] |
| Echinochrome A | Amelioration of intraocular inflammation (uveitis) caused by endotoxins | Lewis rats | [20] |
| Echinochrome A | Decreased risk of atherogenesis and improvement of glutathione metabolism | Human | [21] |
| Echinochrome A | Cardiomyocyte protection against toxic agents | Rat cardiac myoblast H9c2 cells and isolated rat cardiomyocytes. | [1] |
| Echinochrome A | Reduction in diabetic complications in liver | Wistar albino rats | [22] |
| Echinochrome A | Improvement of the musculoskeletal system and the metabolism of lipids and proteins in both types of diabetes mellitus | Wistar albino rats | [23] |
| Echinochrome A | Improvement in the renal function and ameliorating renal histopathological | Winstar albino rats | [3] |
| Echinochrome A | Enhancement in exercise capacity | Sprague–Dawley rats | [24] |
| Echinochrome A | Prevention and/or deceleration of PD-like neurodegeneration | Rats | [25] |
| Echinochrome A | Hepatoprotective effect against intrahepatic cholestasis induced by toxic agents | Rats | [26] |
| Echinochrome A | Enhancing cardiomyocyte differentiation | Mouse embryonic stem cells | [27] |
| Echinochrome A | Potentiating the effectiveness of antitumor therapy | Ehrlich ascites carcinoma model | [28] |
| Echinochrome A | Cardioprotective against the cytotoxicity of doxorubicin | Human cardiomyocyte cell line (AC16) and human breast cancer cell line (MCF-7) | [29] |
| Echinochrome A | Liver antiseptic | Albino rats | [30] |
| Echinochrome A | Improvement in lipid profile, liver functions, kidney functions, and antioxidant markers | Rats | [31] |
| Echinochrome A | Cardioprotective against the cytotoxicity of doxorubicin | AC16 human cardiomyocyte cells | [4] |
| Echinochrome A | Hypolipidemia in obesity | Rats | [31] |
| Echinochrome A | Prevention of atherosclerotic inflammation, SOD3 mimetic, improves the response of the immune system | Human | [12] |
| Echinochrome A | Protective effects on the extracellular matrix of vocal folds in | Ovariectomized rats | [32] |
| Echinochrome A | Antitumor activity, decreases lipid peroxidation, and improves antioxidant status | Ehrlich ascites carcinoma tumor model in mice | [33] |
| Echinochrome A | Anti-inflammatory effect | Rats | [34] |
| Treatment | Effect in CSS | Reference |
|---|---|---|
| Anakinra | IL-1-inhibiting agent | [73] |
| Canakinumab | IL-1-inhibition agent | [54] |
| Rilonacep | IL-1-inhibition agent | [54] |
| Tocilizumab | IL-6-inhibiting agent | [75] |
| Curcumin | Cytokines suppressor | [77] |
| Enzyme CAT | Antioxidant agent: cytokine production regulator through the GSH pathway | [78] |
| N-acetylcysteine (NAC) | IL-6-inhibiting agent through the GSH pathway | [66] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubilar, T.; Barbieri, E.S.; Gazquez, A.; Avaro, M. Sea Urchin Pigments: Echinochrome A and Its Potential Implication in the Cytokine Storm Syndrome. Mar. Drugs 2021, 19, 267. https://doi.org/10.3390/md19050267
Rubilar T, Barbieri ES, Gazquez A, Avaro M. Sea Urchin Pigments: Echinochrome A and Its Potential Implication in the Cytokine Storm Syndrome. Marine Drugs. 2021; 19(5):267. https://doi.org/10.3390/md19050267
Chicago/Turabian StyleRubilar, Tamara, Elena S. Barbieri, Ayelén Gazquez, and Marisa Avaro. 2021. "Sea Urchin Pigments: Echinochrome A and Its Potential Implication in the Cytokine Storm Syndrome" Marine Drugs 19, no. 5: 267. https://doi.org/10.3390/md19050267
APA StyleRubilar, T., Barbieri, E. S., Gazquez, A., & Avaro, M. (2021). Sea Urchin Pigments: Echinochrome A and Its Potential Implication in the Cytokine Storm Syndrome. Marine Drugs, 19(5), 267. https://doi.org/10.3390/md19050267