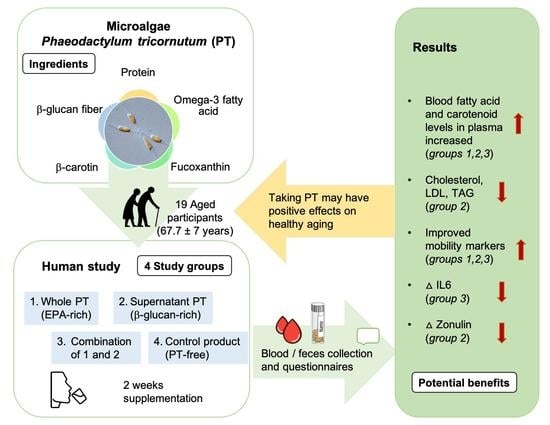
Potentially Beneficial Effects on Healthy Aging by Supplementation of the EPA-Rich Microalgae Phaeodactylum tricornutum or Its Supernatant—A Randomized Controlled Pilot Trial in Elderly Individuals
Abstract
:1. Introduction
2. Results
2.1. Analysis of the Phenolic Content and Oxidative Potential of the Intervention Products
2.2. Clinical Trial-Subjects’ Anthropometric and Metabolic Characteristics at Baseline
2.3. Four-Week Food Diary and Food Frequency Questionnaire (FFQ)
2.4. Acceptance and Adverse Effects during the Intervention
2.5. Effect of Supplementation on Laboratory Parameters
2.6. Mobility Markers and Body Composition before and after Intervention
2.7. Fatty acid Changes in Plasma and Erythrocyte Membrane/Red Blood Cells (RBC)
2.8. Carotenoids, Vitamin E Changes in Plasma
2.9. Inflammatory Parameters and the XOR as an Oxidative Stress Marker
2.10. Gut Barrier Markers and Short-Chain Fatty Acids
2.11. Correlations
3. Discussion
3.1. Safety and Bioavailability
3.2. Potential Antioxidative and Anti-Inflammatory Effects
3.3. Limitations
4. Materials and Methods
4.1. Participant Selection
4.2. Study Design and Outcome Parameters
4.3. Study Products, Randomization and Blinding
4.4. Blood Plasma, Serum and Faecal Measurements
4.5. Antioxidant Assays
4.6. Quantification of Plasma and Erythrocyte Fatty Acids, Carotenoids and Tocopherols
4.7. Inflammatory Markers and Anti-Oxidative Stress Parameter
4.8. Analysis of the Intestinal Permeability Marker Plasma LBP, Faecal Zonulin, and Faecal SCFA
4.9. BIA, Muscle Function Test (5 STS, WOMAC, Gait Speed, Hand Grip Strength)
4.10. Diet Evaluation
4.11. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudnicka, E.; Napierała, P.; Podfigurna, A.; Męczekalski, B.; Smolarczyk, R.; Grymowicz, M. The World Health Organization (WHO) approach to healthy ageing. Maturitas 2020, 139, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Ogra, P.L. Ageing and its possible impact on mucosal immune responses. Ageing Res. Rev. 2010, 9, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Saffrey, M.J. Aging of the mammalian gastrointestinal tract: A complex organ system. Age 2014, 36, 1019–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Rahman, H.S. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martins, D.A.; Custódio, L.; Barreira, L.; Pereira, H.; Ben-Hamadou, R.; Varela, J.; Abu-Salah, K.M. Alternative Sources of n-3 Long-Chain Polyunsaturated Fatty Acids in Marine Microalgae. Mar. Drugs 2013, 11, 2259–2281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryckebosch, E.; Bruneel, C.; Muylaert, K.; Foubert, I. Microalgae as an alternative source of omega-3 long chain polyunsaturated fatty acids. Lipid Technol. 2012, 24, 128–130. [Google Scholar] [CrossRef]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a future food source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef]
- Stiefvatter, L.; Lehnert, K.; Frick, K.; Montoya-Arroyo, A.; Frank, J.; Vetter, W.; Schmid-Staiger, U.; Bischoff, S.C. Oral Bioavailability of Omega-3 Fatty Acids and Carotenoids from the Microalgae Phaeodactylum tricornutum in Healthy Young Adults. Mar. Drugs 2021, 19, 700. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef]
- Stark, K.D.; Van Elswyk, M.E.; Higgins, M.R.; Weatherford, C.A.; Salem, N., Jr. Global survey of the omega-3 fatty acids, docosahexaenoic acid and eicosapentaenoic acid in the blood stream of healthy adults. Prog. Lipid Res. 2016, 63, 132–152. [Google Scholar] [CrossRef]
- Burdge, G. Metabolism of α-linolenic acid in humans. Prostaglandins Leukot. Essent. Fat. Acids 2006, 75, 161–168. [Google Scholar] [CrossRef]
- Calder, P.C. n−3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef] [Green Version]
- Neumann, U.; Louis, S.; Gille, A.; Derwenskus, F.; Schmid-Staiger, U.; Briviba, K.; Bischoff, S.C. Anti-inflammatory effects of Phaeodactylum tricornutum extracts on human blood mononuclear cells and murine macrophages. J. Appl. Phycol. 2018, 30, 2837–2846. [Google Scholar] [CrossRef]
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Neumann, U.; Derwenskus, F.; Gille, A.; Louis, S.; Schmid-Staiger, U.; Briviba, K.; Bischoff, S.C. Bioavailability and Safety of Nutrients from the Microalgae Chlorella vulgaris, Nannochloropsis oceanica and Phaeodactylum tricornutum in C57BL/6 Mice. Nutrients 2018, 10, 965. [Google Scholar] [CrossRef] [Green Version]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Park, H.-A.; Hayden, M.M.; Bannerman, S.; Jansen, J.; Crowe-White, K.M. Anti-Apoptotic Effects of Carotenoids in Neurodegeneration. Molecules 2020, 25, 3453. [Google Scholar] [CrossRef]
- Kim, S.M.; Jung, Y.-J.; Kwon, O.-N.; Cha, K.H.; Um, B.-H.; Chung, D.; Pan, C.-H. A Potential Commercial Source of Fucoxanthin Extracted from the Microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef]
- Fung, A.; Hamid, N.; Lu, J. Fucoxanthin content and antioxidant properties of Undaria pinnatifida. Food Chem. 2013, 136, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Mohibbullah, M.; Haque, M.N.; Khan, M.N.A.; Park, I.-S.; Moon, I.S.; Hong, Y.-K. Neuroprotective effects of fucoxanthin and its derivative fucoxanthinol from the phaeophyte Undaria pinnatifida attenuate oxidative stress in hippocampal neurons. J. Appl. Phycol. 2018, 30, 3243–3252. [Google Scholar] [CrossRef]
- Neumann, U.; Derwenskus, F.; Flaiz Flister, V.; Schmid-Staiger, U.; Hirth, T.; Bischoff, S.C. Fucoxanthin, A Carotenoid Derived from Phaeodactylum tricornutum Exerts Antiproliferative and Antioxidant Activities In Vitro. Antioxidants 2019, 8, 183. [Google Scholar] [CrossRef] [Green Version]
- Grabowska, M.; Wawrzyniak, D.; Rolle, K.; Chomczyński, P.; Oziewicz, S.; Jurga, S.; Barciszewski, J. Let food be your medicine: Nutraceutical properties of lycopene. Food Funct. 2019, 10, 3090–3102. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and Human Health. Pharm. Res. 2007, 55, 207–216. [Google Scholar]
- Chang, C.-S.; Chang, C.-L.; Lai, G.-H. Reactive oxygen species scavenging activities in a chemiluminescence model and neuroprotection in rat pheochromocytoma cells by astaxanthin, beta-carotene, and canthaxanthin. Kaohsiung J. Med. Sci. 2013, 29, 412–421. [Google Scholar] [CrossRef] [Green Version]
- Joshi, Y.B.; Praticò, D. Vitamin E in aging, dementia, and Alzheimer’s disease. BioFactors 2012, 38, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Chen, A.; Zhang, W.; Li, A.; Zhang, C. Co-production of lipids, eicosapentaenoic acid, fucoxanthin, and chrysolaminarin by Phaeodactylum tricornutum cultured in a flat-plate photobioreactor under varying nitrogen conditions. J. Ocean Univ. China 2017, 16, 916–924. [Google Scholar] [CrossRef]
- Stiefvatter, L.; Neumann, U.; Rings, A.; Frick, K.; Schmid-Staiger, U.; Bischoff, S.C. The Microalgae Phaeodactylum tricornutum Is Well Suited as a Food with Positive Effects on the Intestinal Microbiota and the Generation of SCFA: Results from a Pre-Clinical Study. Nutrients 2022, 14, 2504. [Google Scholar] [CrossRef]
- Carballo, C.; Chronopoulou, E.G.; Letsiou, S.; Maya, C.; Labrou, N.E.; Infante, C.; Power, D.; Manchado, M. Antioxidant capacity and immunomodulatory effects of a chrysolaminarin-enriched extract in Senegalese sole. Fish Shellfish Immunol. 2018, 82, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reis, B.; Gonçalves, A.T.; Santos, P.; Sardinha, M.; Conceição, L.E.C.; Serradeiro, R.; Pérez-Sánchez, J.; Calduch-Giner, J.; Schmid-Staiger, U.; Frick, K.; et al. Immune Status and Hepatic Antioxidant Capacity of Gilthead Seabream Sparus aurata Juveniles Fed Yeast and Microalga Derived β-glucans. Mar. Drugs 2021, 19, 653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tang, Y.; Zhang, Y.; Zhang, S.; Qu, J.; Wang, X.; Kong, R.; Han, C.; Liu, Z. Fucoxanthin: A Promising Medicinal and Nutritional Ingredient. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lafarga, T. Effect of microalgal biomass incorporation into foods: Nutritional and sensorial attributes of the end products. Algal Res. 2019, 41, 101566. [Google Scholar] [CrossRef]
- Francezon, N.; Tremblay, A.; Mouget, J.-L.; Pasetto, P.; Beaulieu, L. Algae as a Source of Natural Flavors in Innovative Foods. J. Agric. Food Chem. 2021, 69, 11753–11772. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Xu, D.; Liu, H.; Yang, C.; Xia, H.; Pan, D.; Yang, X.; Yang, L.; Wang, S.; Sun, G. Effects of different delivering matrices of β-glucan on lipids in mildly hypercholesterolaemic individuals: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2020, 125, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Lalia, A.Z.; Lanza, I.R. Insulin-Sensitizing Effects of Omega-3 Fatty Acids: Lost in Translation? Nutrients 2016, 8, 329. [Google Scholar] [CrossRef] [Green Version]
- Shibabaw, T. Omega-3 polyunsaturated fatty acids: Anti-inflammatory and anti-hypertriglyceridemia mechanisms in cardiovascular disease. Mol. Cell. Biochem. 2020, 476, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Côme, M.; Ulmann, L.; Zittelli, G.C.; Faraloni, C.; Nazih, H.; Ouguerram, K.; Chénais, B.; Mimouni, V. Preventive Effects of the Marine Microalga Phaeodactylum tricornutum, Used as a Food Supplement, on Risk Factors Associated with Metabolic Syndrome in Wistar Rats. Nutrients 2019, 11, 1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gora, A.H.; Rehman, S.; Kiron, V.; Dias, J.; Fernandes, J.M.O.; Olsvik, P.A.; Siriyappagouder, P.; Vatsos, I.; Schmid-Staiger, U.; Frick, K.; et al. Management of Hypercholesterolemia Through Dietary SS-glucans–Insights From a Zebrafish Model. Front. Nutr. 2022, 8, 797452. [Google Scholar] [CrossRef]
- Toth, M.J.; Tchernof, A. Lipid metabolism in the elderly. Eur. J. Clin. Nutr. 2000, 54 (Suppl. 3), S121–S125. [Google Scholar] [CrossRef]
- Poli, A.; Corsini, A. Reversible and non-reversible cardiovascular risk in patients treated with lipid-lowering therapy: Analysis of SEAS and JUPITER trials. Eur. J. Intern. Med. 2010, 21, 372–373. [Google Scholar] [CrossRef]
- Nakashima, A.; Yamada, K.; Iwata, O.; Sugimoto, R.; Atsuji, K.; Ogawa, T.; Ishibashi-Ohgo, N.; Suzuki, K. β-Glucan in Foods and Its Physiological Functions. J. Nutr. Sci. Vitaminol. 2018, 64, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Léveillé, P.; Chouinard-Watkins, R.; Windust, A.; Lawrence, P.; Cunnane, S.C.; Brenna, J.T.; Plourde, M. Metabolism of uniformly labeled 13 C-eicosapentaenoic acid and 13 C-arachidonic acid in young and old men. Am. J. Clin. Nutr. 2017, 106, 467–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortier, M.; Tremblay-Mercier, J.; Plourde, M.; Chouinard-Watkins, R.; Vandal, M.; Pifferi, F.; Freemantle, E.; Cunnane, S.C. Higher plasma n-3 fatty acid status in the moderately healthy elderly in southern Québec: Higher fish intake or aging-related change in n-3 fatty acid metabolism? Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 277–280. [Google Scholar] [CrossRef] [Green Version]
- Rees, D.; Miles, E.A.; Banerjee, T.; Wells, S.J.; Roynette, C.E.; Wahle, K.W.; Calder, P.C. Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: A comparison of young and older men. Am. J. Clin. Nutr. 2006, 83, 331–342. [Google Scholar] [CrossRef] [Green Version]
- Bauch, A.; Lindtner, O.; Mensink, G.B.M.; Niemann, B. Dietary intake and sources of long-chain n-3 PUFAs in German adults. Eur. J. Clin. Nutr. 2006, 60, 810–812. [Google Scholar] [CrossRef] [PubMed]
- EPA & DHA Intake Recommendations|GOED Omega-3. Available online: https://goedomega3.com/intake-recommendations (accessed on 21 September 2022).
- Harris, W.S.; Del Gobbo, L.; Tintle, N.L. The Omega-3 Index and relative risk for coronary heart disease mortality: Estimation from 10 cohort studies. Atherosclerosis 2017, 262, 51–54. [Google Scholar] [CrossRef]
- Bresson, J.L.; Flynn, A.; Heinonen, M.; Hulshof, K.; Korhonen, H.; Lagiou, P.; Løvik, M.; Marchelli, R.; Martin, A.; Moseley, B.; et al. Scientific Opinion on the Substantiation of Health Claims Related to Undaria Pinnatifida (Harvey) Suringar and Maintenance or Achievement of a Normal Body Weight (ID 2345) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2009, 7, 1302. [Google Scholar] [CrossRef]
- Bae, M.; Kim, M.-B.; Park, Y.-K.; Lee, J.-Y. Health benefits of fucoxanthin in the prevention of chronic diseases. Biochim. Biophys. Acta BBA-Mol. Cell Biol. Lipids 2020, 1865, 158618. [Google Scholar] [CrossRef]
- Lee, A.-H.; Shin, H.-Y.; Park, J.-H.; Koo, S.Y.; Kim, S.M.; Yang, S.-H. Fucoxanthin from microalgae Phaeodactylum tricornutum inhibits pro-inflammatory cytokines by regulating both NF-κB and NLRP3 inflammasome activation. Sci. Rep. 2021, 11, 543. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, O.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Kumar, M.; Babaei, P.; Ji, B.; Nielsen, J. Human gut microbiota and healthy aging: Recent developments and future prospective. Nutr. Health Aging 2016, 4, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K. Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Available online: https://www.hindawi.com/journals/mi/2021/8879227/ (accessed on 18 February 2021).
- Qi, Y.; Goel, R.; Kim, S.; Richards, E.M.; Carter, C.S.; Pepine, C.J.; Raizada, M.K.; Buford, T.W. Intestinal Permeability Biomarker Zonulin is Elevated in Healthy Aging. J. Am. Med. Dir. Assoc. 2017, 18, 810.e1–810.e4. [Google Scholar] [CrossRef]
- Rico, M.; López, A.; Santana-Casiano, J.M.; Gonzàlez, A.G.; Gonzàlez-Dàvila, M. Variability of the phenolic profile in the diatom Phaeodactylum tricornutum growing under copper and iron stress. Limnol. Oceanogr. 2013, 58, 144–152. [Google Scholar] [CrossRef]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (αTEAC), DPPH assay and peroxyl radical scavenging assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- De Alencar, D.B.; Diniz, J.C.; Rocha, S.A.; Pires-Cavalcante, K.M.; De Lima, R.L.; De Sousa, K.C.; Freitas, J.O.; Bezerra, R.M.; Baracho, B.M.; Sampaio, A.H.; et al. Fatty acid composition from the marine red algae Pterocladiella capillacea (S. G. Gmelin) Santelices & Hommersand 1997 and Osmundaria obtusiloba (C. Agardh) R. E. Norris 1991 and its antioxidant activity. An. Acad. Bras. Ciênc. 2018, 90, 449–459. [Google Scholar] [CrossRef]
- Meijer, B.; Seinen, M.L.; Hosman, T.; Linskens, R.K.; Kneppelhout, J.-K.; Peters, G.J.; Mulder, C.J.; Van Bodegraven, A.A.; De Boer, N.K. High inter-individual variability of serum xanthine oxidoreductase activity in IBD patients. Nucleosides Nucleotides Nucleic Acids 2018, 37, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Aranda, R.; Doménech, E.; Rus, A.D.; Real, J.T.; Sastre, J.; Vina, J.; Pallardó, F.V. Age-related increase in xanthine oxidase activity in human plasma and rat tissues. Free Radic. Res. 2007, 41, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a Marine Carotenoid Present in Brown Seaweeds and Diatoms: Metabolism and Bioactivities Relevant to Human Health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef]
- Lepretti, M.; Martucciello, S.; Burgos Aceves, M.A.; Putti, R.; Lionetti, L. Omega-3 Fatty Acids and Insulin Resistance: Focus on the Regulation of Mitochondria and Endoplasmic Reticulum Stress. Nutrients 2018, 10, 350. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, S.; Gato, A.; Lamela, M.; Freire-Garabal, M.; Calleja, J.M. Anti-inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother. Res. 2003, 17, 665–670. [Google Scholar] [CrossRef]
- Tan, A.; Sullenbarger, B.; Prakash, R.; McDaniel, J.C. Supplementation with eicosapentaenoic acid and docosahexaenoic acid reduces high levels of circulating proinflammatory cytokines in aging adults: A randomized, controlled study. Prostaglandins Leukot. Essent. Fat. Acids 2018, 132, 23–29. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Smith, G.I.; Atherton, P.; Reeds, D.N.; Mohammed, B.S.; Rankin, D.; Rennie, M.J.; Mittendorfer, B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: A randomized controlled trial. Am. J. Clin. Nutr. 2011, 93, 402–412. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.I.; Julliand, S.; Reeds, D.N.; Sinacore, D.R.; Klein, S.; Mittendorfer, B. Fish oil–derived n−3 PUFA therapy increases muscle mass and function in healthy older adults. Am. J. Clin. Nutr. 2015, 102, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, C.H.; Flanagan, E.M.; De Vito, G.; Susta, D.; Mitchelson, K.A.J.; Castro, E.D.M.; Senden, J.M.G.; Goessens, J.P.B.; Mikłosz, A.; Chabowski, A.; et al. Does supplementation with leucine-enriched protein alone and in combination with fish-oil-derived n–3 PUFA affect muscle mass, strength, physical performance, and muscle protein synthesis in well-nourished older adults? A randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr. 2021, 113, 1411–1427. [Google Scholar] [CrossRef] [PubMed]
- Kalstad, A.A.; Myhre, P.L.; Laake, K.; Tveit, S.H.; Schmidt, E.B.; Smith, P.; Nilsen, D.W.T.; Tveit, A.; Fagerland, M.W.; Solheim, S.; et al. Effects of n-3 Fatty Acid Supplements in Elderly Patients after Myocardial Infarction: A Randomized Controlled Trial. Circulation 2020, 143, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Sahni, S.; Dufour, A.B.; Fielding, R.A.; Newman, A.B.; Kiel, D.P.; Hannan, M.T.; Jacques, P.F. Total carotenoid intake is associated with reduced loss of grip strength and gait speed over time in adults: The Framingham Offspring Study. Am. J. Clin. Nutr. 2020, 113, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Song, C.; Song, X.; Zhang, X.; Li, X. Carotenoids and risk of fracture: A meta-analysis of observational studies. Oncotarget 2016, 8, 2391–2399. [Google Scholar] [CrossRef] [Green Version]
- Eftekharsadat, B.; Aghamohammadi, D.; Dolatkhah, N.; Hashemian, M.; Salami, H. Lower serum levels of alpha tocopherol and lycopene are associated with higher pain and physical disability in subjects with primary knee osteoarthritis: A case-control study. Int. J. Vitam. Nutr. Res. 2021, 91, 304–314. [Google Scholar] [CrossRef]
- Power, R.; Nolan, J.M.; Prado-Cabrero, A.; Roche, W.; Coen, R.; Power, T.; Mulcahy, R. Omega-3 fatty acid, carotenoid and vitamin E supplementation improves working memory in older adults: A randomised clinical trial. Clin. Nutr. 2021, 41, 405–414. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products; Nutrition and Allergies (NDA). Scientific Opinion on the Safety of ‘Yeast Beta-glucans’ as a Novel Food Ingredient. EFSA J. 2011, 9, 2137. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wendlinger, C.; Hammann, S.; Vetter, W. Various concentrations of erucic acid in mustard oil and mustard. Food Chem. 2014, 153, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Thurnhofer, S.; Vetter, W. A Gas Chromatography/Electron Ionization−Mass Spectrometry−Selected Ion Monitoring Method for Determining the Fatty Acid Pattern in Food after Formation of Fatty Acid Methyl Esters. J. Agric. Food Chem. 2005, 53, 8896–8903. [Google Scholar] [CrossRef]
- HS-Omega-3 Index-Omegametrix. Available online: https://www.omegametrix.eu/hs_omega_3_index.php (accessed on 19 September 2022).
- Stuetz, W.; McGready, R.; Cho, T.; Prapamontol, T.; Biesalski, H.; Stepniewska, K.; Nosten, F. Relation of DDT residues to plasma retinol, α-tocopherol, and β-carotene during pregnancy and malaria infection: A case–control study in Karen women in northern Thailand. Sci. Total Environ. 2006, 363, 78–86. [Google Scholar] [CrossRef]
- Montoya-Arroyo, A.; Toro-González, C.; Sus, N.; Warner, J.; Esquivel, P.; Jiménez, V.M.; Frank, J. Vitamin E and carotenoid profiles in leaves, stems, petioles and flowers of stinging nettle (Urtica leptophylla Kunth) from Costa Rica. J. Sci. Food Agric. 2022, 102, 6340–6348. [Google Scholar] [CrossRef] [PubMed]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gomez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.E.; Kon, S.S.C.; Canavan, J.L.; Patel, M.S.; Clark, A.L.; Nolan, C.M.; Polkey, M.I.; Man, W.D.-C. The five-repetition sit-to-stand test as a functional outcome measure in COPD. Thorax 2013, 68, 1015–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McConnell, S.; Kolopack, P.; Davis, A. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): A review of its utility and measurement properties. Arthritis Care Res. 2001, 45, 453–461. [Google Scholar] [CrossRef]
- Haftenberger, M.; Heuer, T.; Heidemann, C.; Kube, F.; Krems, C.; Mensink, G.B. Relative validation of a food frequency questionnaire for national health and nutrition monitoring. Nutr. J. 2010, 9, 36. [Google Scholar] [CrossRef]








| All (n = 19) | Comp (n = 5) | A (n = 5) | SupB (n = 5) | A+SupB (n = 4) | |
|---|---|---|---|---|---|
| Female/Male [n] | 12/7 | 2/3 | 4/1 | 3/2 | 3/1 |
| Anthropometry | |||||
| Age [years] | 67.7 ± 6.5 | 67.4 ± 7.9 | 65.4 ± 4.7 | 71.4 ± 5.7 | 67 ± 9.2 |
| BMI [kg/m²] | 24.6 ± 3.1 | 26.9 ± 2.5 | 25.5 ± 1.8 | 22.3 ± 2.9 | 23.3 ± 3.7 |
| Waist circumference [cm] | 90.2 ± 11.6 | 100.5 ± 8.7 | 93.6 ± 4.5 | 81.2 ± 11.4 | 84.5 ± 11.2 |
| Blood biomarkers | |||||
| Cholesterol (chol.) [mg/dl] | 224.6 ± 50.0 | 248 ± 43.5 | 198 ± 44.7 | 219 ± 63.2 | 235 ± 46.5 |
| Triglycerides [mg/dl] | 99.9 ± 60.0 | 141 ± 102.1 | 83.2 ± 35.7 | 74 ± 26.7 | 101.8 ± 26.5 |
| HDL-chol. [mg/dl] | 71.1 ± 18.4 | 71.8 ± 24.2 | 64 ± 14 | 72.2 ± 12 | 77.8 ± 25.7 |
| LDL-chol. [mg/dl] | 126.4 ± 46.8 | 152.2 ± 24.1 | 120.2 ± 40.9 | 129.8 ± 51.2 | 129.7 ± 11.7 |
| Insulin [µE/mL] | 7.8 ± 4.1 | 10.8 ± 6.6 | 7.6 ± 2.8 | 5.9 ± 2.6 | 6.7 ± 1.5 |
| HOMA-Index | 1.9 ± 1.7 | 3.1 ± 3 | 1.7 ± 0.6 | 1.4 ± 0.6 | 1.5 ± 0.2 |
| Side Effects | Comp (n = 5) Diary/Protocol | A (n = 5) Diary/Protocol | SupB (n = 5) Diary/Protocol | A+SupB (n = 4) Diary/Protocol | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min. | Mild | Sev. | Min. | Mild | Sev. | Min. | Mild | Sev. | Min. | Mild | Sev. | |
| Abdominal rumbling | - | - | - | 2│11 | - | - | - | - | - | - | - | - |
| Flatulence | - | 1│02 | - | - | - | - | - | 1│13 | - | - | 1│0 | - |
| Stomach pain | - | - | - | - | - | - | - | 0│1 | - | - | - | - |
| Diarrhoea | - | 1│1 | - | - | - | - | - | 1│0 | - | - | 1│0 | - |
| Discoloration of the stool | - | - | - | - | - | - | - | - | - | 1│0 | - | - |
| Decreased frequency of bowel movements | - | - | - | - | - | - | - | - | - | 1│0 | - | - |
| Belching (at least 1×) | - | - | - | - | - | - | - | - | - | 1│1 | - | - |
| Headache | - | - | - | - | - | - | - | - | - | 1│0 | 1│0 | - |
| Increased blood pressure | - | 1│0 | - | - | - | - | - | - | - | - | - | - |
| Increased urge to urinate | - | - | - | - | - | - | - | - | - | 1│0 | - | - |
| Nausea | - | - | - | 1│1 | - | - | - | - | - | - | - | - |
| Daily Nutrient Intake | Comp | A | SupB | A+SupB |
|---|---|---|---|---|
| Protein g/day | 0.007 | 0.81 | 0.05 | 0.85 |
| β-1,3-glucan g/day | 0 | 0.17 | 0.54 | 0.71 |
| FAs [mg/day] | 13.02 | 678.3 | 22.9 | 688.2 |
| n−3 FA | 0.34 | 312.51 | 1.79 | 313.97 |
| n−6 FA | 5.19 | 63.56 | 5.57 | 63.94 |
| SFA | 3.69 | 90.85 | 7.35 | 94.51 |
| MUFA | 3.80 | 104.38 | 7.70 | 108.28 |
| PUFA | 5.53 | 483.07 | 7.85 | 485.38 |
| n−6: n−3 ratio | 20.00 | 20.43 | 20.47 | 20.90 |
| AA/EPA ratio | 0.00 | 0.22 | 0.00 | 0.22 |
| EPA+DHA | 0.03 | 293.65 | 1.20 | 294.82 |
| EPA | 0.02 | 288.52 | 1.44 | 289.94 |
| DHA | 0.01 | 5.13 | 0.06 | 5.19 |
| Carotenoids [mg/day] | ||||
| Fucoxanthin | 0.00 | 21.39 | 0.22 | 21.61 |
| β-carotene | 0.01 | 0.29 | 0.01 | 0.30 |
| α-carotene | 0.00 | 0.00 | 0.00 | 0.00 |
| Lycopene | 0.00 | 0.21 | 0.01 | 0.21 |
| Vitamine E [mg/day] | ||||
| α-Tocopherol | 0.00 | 0.10 | 0.03 | 0.13 |
| β-Tocopherol | 0.00 | 0.00 | 0.00 | 0.00 |
| γ-Tocopherol | 0.00 | 0.01 | 0.00 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stiefvatter, L.; Frick, K.; Lehnert, K.; Vetter, W.; Montoya-Arroyo, A.; Frank, J.; Schmid-Staiger, U.; Bischoff, S.C. Potentially Beneficial Effects on Healthy Aging by Supplementation of the EPA-Rich Microalgae Phaeodactylum tricornutum or Its Supernatant—A Randomized Controlled Pilot Trial in Elderly Individuals. Mar. Drugs 2022, 20, 716. https://doi.org/10.3390/md20110716
Stiefvatter L, Frick K, Lehnert K, Vetter W, Montoya-Arroyo A, Frank J, Schmid-Staiger U, Bischoff SC. Potentially Beneficial Effects on Healthy Aging by Supplementation of the EPA-Rich Microalgae Phaeodactylum tricornutum or Its Supernatant—A Randomized Controlled Pilot Trial in Elderly Individuals. Marine Drugs. 2022; 20(11):716. https://doi.org/10.3390/md20110716
Chicago/Turabian StyleStiefvatter, Lena, Konstantin Frick, Katja Lehnert, Walter Vetter, Alexander Montoya-Arroyo, Jan Frank, Ulrike Schmid-Staiger, and Stephan C. Bischoff. 2022. "Potentially Beneficial Effects on Healthy Aging by Supplementation of the EPA-Rich Microalgae Phaeodactylum tricornutum or Its Supernatant—A Randomized Controlled Pilot Trial in Elderly Individuals" Marine Drugs 20, no. 11: 716. https://doi.org/10.3390/md20110716
APA StyleStiefvatter, L., Frick, K., Lehnert, K., Vetter, W., Montoya-Arroyo, A., Frank, J., Schmid-Staiger, U., & Bischoff, S. C. (2022). Potentially Beneficial Effects on Healthy Aging by Supplementation of the EPA-Rich Microalgae Phaeodactylum tricornutum or Its Supernatant—A Randomized Controlled Pilot Trial in Elderly Individuals. Marine Drugs, 20(11), 716. https://doi.org/10.3390/md20110716