A Systematic Review on Marine Algae-Derived Fucoxanthin: An Update of Pharmacological Insights
Abstract
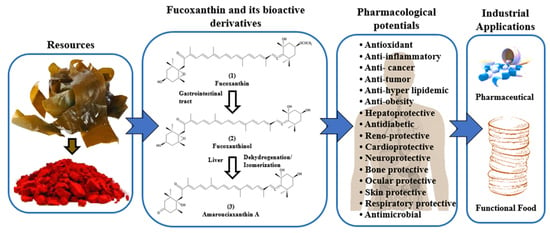
:1. Introduction
2. Results and Discussion
2.1. Current Research Trends on Fucoxanthin
2.2. Structural Characteristics of Fucoxanthin
2.3. Pharmacological Properties of Fucoxanthin Evidence from In Vitro and In Vivo Studies
2.3.1. Antioxidant Activity
| Experimental Model (In Vitro/In Vivo) | Treatment (Dose, Route and Duration) | Major Outcomes | Reference |
|---|---|---|---|
| In vitro cell-free assays | 0.01–2 mg/mL extracted from F. vesiculosus, F. serratus, and L. digitata in 5% fish oil in water emulsion; butylated hydroxytoluene as positive control | ↑ DPPH scavenging and iron-chelating activity; ↓ reducing power | [30] |
| LPS-induced RAW 264.7 and HepG2, Caco-2 and HeLa cells | 0.1–50 μg/mL (purity ≥ 99.2%) extracted from P. tricornutum in 0.1% DMSO, pre-treatment for 24 h; staurosporine 1 μM as positive control | ↑ DPPH activity with IC50 value of 201.2 ± 21.4 µg/mL ↓ metabolic activity and caspase 3/7 | [7] |
| OVA-induced-asthma mouse | 50 mg/kg b.w., treatment (N/A) | ↓ ROS; ↑ antioxidant enzyme activity; ↓ inflammatory cytokine markers | [32] |
| Alcohol-induced liver injury in mice | 10–40 mg/kg, orally for 7 days; silibinin 80 mg/kg as positive control | ↑ T-AOC, GSH-Px, SOD and CAT; ↑ Nrf2, NQO1, HO-1 and GCLM | [9] |
| 4-HNE induced-diabetic retinopathy in ARPE-19 cells | 0.1–0.5 mg/mL, post-treatment for 24 or 72 h | ↑ Cell viability; ↓ DNA damage; ↓ cleaved PARP; Nrf2 protein; ↓ ICAM-1 protein expression; ↑ ZO-1 expression; ↓ ROS; ↑ CAT | [23] |
| In vitro cell-free assays | 0.05–0.3 mg/mL extracted from Isochrysis galbana | ↑ DPPH activity with EC50 value of 0.2 mg/mL | [31] |
| UVA-induced reconstructed human skin tissue | 0.5% extracted from D. anceps, pre-treatment for 1 h | ↓ intracellular ROS | [34] |
| LPS-induced uveitis in rats | 1–10 mg/kg b.w. in 0.1% DMSO, Orally for 7 days | ↑ Nrf2 in ocular tissues; ↑ SOD; ↓ MDA | [33] |
| TGFβ1-induced fibrosis in human LX-2 cells | FxOH 0.1–0.5 μM (purity ≥ 97%) and AcxA 0.2–1 μM (purity ≥ 97%) in DMSO, pre-treatment for 1–24 h | ↓ ROS; ↑ Nrf2 expression | [6] |
| Cadmium-induced thyroid gland injury mice | 10–50 mg/kg b.w., orally for 14 days; thyroid tablets 50 mg/kg as positive control | ↑ POD, SOD, CAT and APX; ↓ mRNA expressions of ERK1 and 2, caspase3, 8 and 9 | [8] |
2.3.2. Anti-Inflammatory Activity
| Experimental Model (In Vitro/In Vivo) | Treatment (Dose, Route, and Duration) | Major Outcomes | Reference |
|---|---|---|---|
| LPS-activated BV-2 microglia | 5–20 μM (purity ≥ 98%), pre-treatment for 1 h | ↓ IL-6, TNF-α, PGE2, NO, iNOS, COX-2 enzymes; ↑ Nrf-2 activation; ↑ HO-1 expression; ↑ BDNF; ↓ Akt/NF-κB; ↓ MAPKs/AP-1 | [11] |
| UV-B-stimulated corneal denervation in rats | 0.1 to 10 mg/kg b.w., orally for 6 days | ↑ Nrf2 in cornea; ↓ p38 MAPK; ↓ GFAP-positive neural cells; ↓ TRPV1 expression in the trigeminal ganglia neurons | [42] |
| LPS-treated mice | 50–200 mg/kg b.w. in 0.5% sodium carboxymethylcellulose, intragastric route for 7 days | ↑ AMPK; ↓ NF-κB; ↓ TNF-α, IL-1β, IL-6; ↓ iNOS and COX-2 | [41] |
| LPS-induced sepsis mouse model | 0.1–10 mg/kg b.w. extracted from Conticribra weissflogii ND-8, intraperitoneally for 6–120 h; ulinastatin as positive control | ↓ IL-6, IL-1β and TNF-α; | [44] |
| LPS-induced RAW 264.7 cells | 10 nM extracted from Conticribra weissflogii ND-8, co-treatment for 6 h | ↓ NF-κB signaling pathway | |
| Palmitate-activated RAW 264.7 cells | 50 μM (purity ≥ 95%), co-treatment for 12 h | ↓ IL-6, IL-1β, TNF-α and NLRP3 gene; ↑ TGF β gene; ↑ CPT1a, PPAR γ; ↑ pAMPK | [36] |
| CDAHFD-induced NASH model mice | 0.2%/day extracted from brown seaweed lipid, orally for 4 weeks | ↓ Hepatic IL-1β, IL-6, TNF-α mRNA expression; ↓ MCP-1 mRNA expression; ↓ serum MCP-1 | [37] |
| UVA-induced reconstructed human skin | all-trans fucoxanthin (0.5% w/v) (purity ≥ 95%) in Alkyl benzoate and ethanol, co-treatment for 15 min; sodium dodecyl sulfate as positive control | ↓ IL-6, IL-8 gene expression | [39] |
| DSS-stimulated ulcerative colitis mice | 50–100 mg/kg b.w., treatment (NA) | ↓ PGE2, COX-2; ↓ NF-κB | [10] |
| LPS-induced RAW 264.7 macrophages | 4.7–470 ng/mL (purity ≥ 95%) extracted from T. lutea F&M-M36, co-treatment for 18 h; celecoxib 3 μM as positive control | ↓ IL-6; ↑ IL-10, Arg1 | [38] |
| PM-induced zebrafish embryo | 25–100 μg/mL extracted from Sargassum fusiformis, co-treatment for 72 h | ↓ NO, ROS | [40] |
| PM-activated HaCaT keratinocytes and RAW 264.7 cells | 25–100 μg/mL extracted from Sargassum fusiformis, co-treatment for 30 min | ↓ NO, IL-1β, TNF-α and IL-6; ↓ PGE2, COX-2 and MAPK | |
| LPS-activated RAW 264.7 cells | 5 μM (purity ≥ 95%), pre-treatment for 12 h | ↓ IL6, IL-1β and TNF mRNA; ↓ TNFα secretion; ↓ PI3K/AKT/ Nrf2 | [35] |
| LPS/ATP-stimulated BMDMs and BMDCs | 40 μM extracted from Phaeodactylum tricornutum, pre-treatment for 4 h | ↓ IL-1β, IL-6 and TNF-α; ↓ NLRP3, ASC and cleaved caspase-1; ↓ oligomerization of ASC; ↓ NF-κB | [45] |
| LPS-induced RAW264.7 cells | 2.5 μM (purity ≥ 96%) fucoxanthinol from fucoxanthin, extracted from brown seaweed lipid, co-treatment for 24 h | ↓ proinflammatory mediators; ↓ MAPK/NF-κB signaling pathways | [46] |
| OVA-triggered asthmatic mice | 10–30 mg/kg b.w. (purity ≥ 95%) in DMSO, intraperitoneally for 28 days; prednisolone 5 mg/kg as positive control | ↓ IL-8, MCP-1 and CCL5; ↓IL-4, IL-5, IL-13; ↑ IFN-γ expression | [43] |
2.3.3. Anticancer and Anti-Tumor Activity
| Experimental Model (In Vitro/In Vivo) | Treatment (Dose, Route and Duration) | Major Outcomes | Reference |
|---|---|---|---|
| CCSCs, CD44high/EpCAMhigh tumor cells and HT-29 human colorectal cancer cells | 0.1–5.0 µM fucoxanthinol from fucoxanthin (purity ≥ 98%) in tetrahydrofuran, treatment for 5 days | ↓ Cells viabilities; ↓ pAkt, PPARβ/δ and PPARγ; ↓ Colonospheres growth; ↑ Chromatin condensation; ↑ Nuclear fragmentations | [48] |
| NOD-SCID mice with tumors | 5 mg/kg b.w. fucoxanthinol from fucoxanthin (purity ≥ 98%) in tetrahydrofuran, orally every 3–4 days for 2 weeks | ↓ Csps tumorigenesis | |
| Leukemia cell lines, K562 and TK6 | 0.1–10 μM in DMSO, treatment for 24 h | ↓ Cell viability and proliferation; ↓ Nuclei size; ↓ Anti-apoptotic protein (bcl-2 and caspase-3) | [13] |
| Breast cancer cells line, MDA-MB-231 and normal human skin fibroblast cells line | 10–50 μg/mL extracted from P. tenuis, C. sinuosa, I. stellate and D. indica in DMSO, treatment for 6–48 h | ↑ Death of cancer cells; ↓ Cell viability | [51] |
| Human gastric adenocarcinoma SGC-7901 or BGC-823 cells | 25–75 μM (purity ≥ 99%) extracted from Undaria pinnatifda in ethanol, pre-treatment for 24 h; paclitaxel 1 μM as positive control | ↑ Apoptotic cells; ↓ Cells cycle at S phase (SGC-7901) and G2/M phase (BGC-823); ↓ Mcl-1, STAT3 and p-STAT3 | [53] |
| Benzo(A)pyrene-induced lung cancer mice | N/A | ↑ Apoptosis (Caspase 9 and 3); ↓ Anti-apoptotic protein (Bcl2); ↓ Expression of PCNA | [54] |
| Human liver HepG2 cancer cell line | 10-40 μgmL−1 extracted from Chaetoceros calcitrans in DMSO, treatment for 72 h; doxorubicin as positive control | ↓ Proliferation; ↓ AKT1, ERK ½, JNK expression; ↑ BAX and BID gene; ↑ APAF and CYCS expression; ↓ Antioxidant genes (SOD1, SOD2, CAT) | [55] |
| Human breast cancer MDA-MB-231 cells | 25–100 μM extracted from U pinnatifida, treatment for 12–48 h | ↓ Lymphangiogenesis; ↓ VEGF-C, VEGF receptor-3, NF-κB, p-Akt and p-PI3K, micro-LVD | [12] |
| GBM1, A172 and C6 cell lines | 10–150 μM extracted from Phaeodactylum tricornutum, treatment for 24 h | ↓ Cell viability and proliferation and invasion; ↓ Angiogenesis and tubulogenesis; ↓ ATP levels; ↑ Apoptosis | [52] |
| AOM/DSS-induced carcinogenic mice | 30 mg/kg b.w. in palm oil, orally every 1 or 3 days for 3 weeks | ↑ Mucosal crypts and anoikis-like integrin 1low/-/cleaved caspase-3high cells; ↓ Integrin阝, pFAK, pPaxillin, αSMA | [14] |
| HeLa and SiHa cervical cancer cells | 0.1–25 µM, treatment for 48 h | ↓ Hela and SiHa cells (IC50: 1445 and 1641 µM, respectively) ↑ apoptosis; ↓ cell proliferation and colony formation; ↓ HIST1H3D and its mRNA, cell cycle at G0/G1 phase | [56] |
| Human non-small cell lung cancer A549, H1299, PC9 and small cell lung cancer H446 cell lines | 5–30 μM (purity ≥ 99%) extracted from Laminaria Japonica in ethanol, treatment for 48 h; diamminedichloroplatinum 5 mg/kg as positive control | ↓ Cells migration and invasion, metastasis; ↓ Expressions of Snail, Twist, Fibronectin, N-cadherin, MMP-2, PI3K, p-AKT and NF-Κb; ↑ Expression of TIMP-2 | [60] |
| C57BL/6J mice, orthotopic transplantations of cancer cells (KMPC44) | 3 mg/g b.w. in palm oil, orally for 2 weeks | ↓ Adenocarcinoma; ↓ CCL21/ CCR7 axis, Rho A, BTLA, N-cadherin, SMA, pFAK and pPaxillin | [57] |
| AOM/DSS-induced colorectal tumorigenesis in ApcMin/+ mice | 30 mg/kg b.w., orally for 5 weeks | ↑ Cleaved caspase-3; ↓ cyclin D1 expression; ↓ Bacteroidlales and Rikenellaceae; ↑ Lachnospiraceae | [49] |
| AOM/DSS-induced colorectal cancer mice | 50 mg/kg b.w., orally for 14 weeks | ↓ Ccr1, Cyclin D1, pSmad2, MAPK, PI3K/AKT, p53, RAS, STAT, TGF-β and Wnt | [50] |
| TPA-induced skin cell transformation in Nfe2l2wild-type cells | N/A | ↓ ROS, oxidized GSSG/reduced GSH | [58] |
2.3.4. Anti-Hyperlipidemic and Anti-Obesity Potentials
2.3.5. Antidiabetic Activity
2.3.6. Cardioprotective Activity
2.3.7. Hepatoprotective Activity
2.3.8. Reno-Protective Activity
2.3.9. Ocular Protective Activity
2.3.10. Neuroprotective Activity
| Experimental Model (In Vitro/In Vivo) | Treatment (Dose, Route and Duration) | Major Outcomes | Reference |
|---|---|---|---|
| β-Amyloid oligomer-induced neurotoxicity in SH-SY5Y Cells | 0.3–3 μM extracted from Sargassum horneri (purity ≥ 90%), pre-treatment for 2 h | ↓ neuronal loss and oxidative stress; ↓ ROS; ↑ pAkt and pGSK3β; ↓ pERK | [84] |
| H2O2-induced toxicity in SH-SY5Y Cells and primary cerebellar granule neurons | 0.3–3 μM extracted from Sargussum horneri (purity ≥ 90%), pre-treatment for 2 h | ↓ neuronal apoptosis and oxidative stress; ↓ ROS; ↑ pAkt and pGSK3β; ↓ pERK; | [85] |
| Aβ1–42 oligomers-induced neurotoxicity in SH-SY5Y Cells | 0.1–1 μM extracted from Sargussum horneri (purity ≥ 90%), co-treatment for 24 h | ↑ cell viability | [18] |
| Aβ oligomer-induced cognitive impairments in mice | 50−200 mg/kg b.w. extracted from Sargussum horneri (purity ≥ 90%) in sterile saline, orally for 17 days | ↑ memory formation; ↓ oxidative stress; ↑ SOD, CAT and GSH Activities; ↑ BDNF and ChAT | |
| Scratch-injury in cortical neurons | 5–20 μM (purity ≥ 95%) in DMSO, post-treatment for 1 day | ↓ MDA, GPx, ROS; ↑ viability | [91] |
| TBI-employed mice | 50–200 mg/kg b.w. (purity ≥ 95%) in olive oil, orally for 1–7 days; 0.01–0.1 mmol/L, intracerebroventricular injection for 1–7 days | ↑ Nrf2-ARE expression | |
| OGD/R- induced apoptosis neurons | 5–20 μM (purity ≥ 95%) in DMSO, pre-treatment for 30 h | ↓ Apoptosis, ROS, MDA; ↑ SOD; ↓ Cleaved caspase-3; ↑ Bcl-2/Bax expression; ↑ Nrf2 and HO-1 expression | [90] |
| MCAO-induced rat model (cerebral I/R injury) | 30–90 mg/kg (purity ≥ 95%) in DMSO, intragastrically, 1 h before MCAO | ↑ SOD activity; ↓ ROS and MDA; ↓ cleaved caspase-3; ↑ Bcl-2/Bax ratio | |
| H/R-induced excitotoxicity in primary hippocampal neurons | 0.025–0.25 μg/mL extracted from Undaria pinnatifida in DMSO, co-treatment for 1.5 h of hypoxia and 24 h of reoxygenation | ↑ viability; ↑ length of primary neurites | [19] |
| Aβ1-42- and H2O2-mediated cytotoxicity in PC12 cells | 0.01–2 μM (purity ≥ 95%) in DMSO, pre-treatment for 15 min | ↑ cell viability; ↓ apoptosis | [86] |
| Aβ oligomers-induced neurotoxicity in SH-SY5Y cells and LPS- induced neuro-inflammation in BV2 cells | PLGA-PEGFuc nanoparticles (1-10 μg/mL in 0.1% Tween-80), extracted from Sargussum horneri (purity ≥ 90%), co-treatment for 2 h | ↑ viability; ↓ ROS; ↓ IL-1β and TNF-α | [88] |
| Aβ oligomers-induced recognition impairments in mice | PLGA-PEGFuc nanoparticles (i.v. 20–50 mg/kg b.w. in 0.1% Tween-80), extracted from Sargussum horneri (purity ≥ 90%), intravenous injection in every 2 days for 3 times | ↑ cognitive performance; ↑ Nrf2; ↓ NF-κB; ↓ IL-1β and TNF-α; ↑ SOD and CAT | |
| Intracerebroventricular streptozotocin (ICV-STZ)-induced cognitive impairment in rats | 50–100 mg/kg b.w., orally for 14 days | ↑ cognitive performance; ↓ MDA and nitrite; ↑ GSH, SOD and CAT; ↓ TNF-α, IL-1β and IL-6; ↓ Aβ(1–42) and Tau accumulation | [87] |
| 6-OHDA-induced neurotoxicity in PC12 cells | 0.5–5 μM in DMSO, pre-treatment for 2 h | ↓ apoptosis; ↑ HO-1, GCLM and GCLC levels; ↑ Nrf2; ↓ Keap1 | [89] |
| 6-OHDA-exposed zebrafish | 6.25–50 μg/mL in DMSO, pre-treatment for 2 h + incubation for 4 days after 6-OHDA exposure | ↑ swimming capacity; ↓ brain tissue damage; ↓ ROS |
2.3.11. Bone Protective Activity
2.3.12. Respiratory Protective Activity
2.3.13. Skin Protective Activity
2.3.14. Antimicrobial Activity
2.3.15. Other Bioactivities
3. Pharmacokinetics of Fucoxanthin
4. Safety, Toxicity, and Functional Stability of Fucoxanthin
5. Clinical Perspectives of Fucoxanthin
6. Pharmaceutical Prospects of Fucoxanthin
7. Materials and Methods
7.1. Literature Search
7.2. Selection Criteria
7.3. Data Extraction
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Khaw, Y.S.; Yusoff, F.M.; Tan, H.T.; Noor Mazli, N.A.I.; Nazarudin, M.F.; Shaharuddin, N.A.; Omar, A.R. The critical studies of fucoxanthin research trends from 1928 to june 2021: A bibliometric review. Mar. Drugs 2021, 19, 606. [Google Scholar] [CrossRef] [PubMed]
- Aman, R.; Schieber, A.; Carle, R. Effects of heating and illumination on trans-cis isomerization and degradation of beta-carotene and lutein in isolated spinach chloroplasts. J. Agric. Food Chem. 2005, 53, 9512–9518. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.L.; Sudirman, S.; Hsu, Y.C.; Su, C.Y.; Kuo, H.P. Fucoxanthin-rich brown algae extract improves male reproductive function on streptozotocin- nicotinamide-induced diabetic rat model. Int. J. Mol. Sci. 2019, 20, 4485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharova, L.V.; Daurtseva, A.V.; Flisyuk, E.V.; Shikov, A.N. Efficacy of Natural Deep Eutectic Solvents for Extraction of Hydrophilic and Lipophilic Compounds from Fucus vesiculosus. Molecules 2021, 26, 4198. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Fraga-Corral, M.; Jimenez-Lopez, C.; Carpena, M.; Pereira, A.G.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Biological action mechanisms of fucoxanthin extracted from algae for application in food and cosmetic industries. Trends Food Sci. Technol. 2021, 117, 163–181. [Google Scholar] [CrossRef]
- Li, Y.; Kim, M.B.; Park, Y.K.; Lee, J.Y. Fucoxanthin metabolites exert anti-fibrogenic and antioxidant effects in hepatic stellate cells. J. Agric. Food Res. 2021, 6, 100245. [Google Scholar] [CrossRef]
- Neumann, U.; Derwenskus, F.; Flister, V.F.; Schmid-Staiger, U.; Hirth, T.; Bischoff, S.C. Fucoxanthin, a carotenoid derived from Phaeodactylum tricornutum exerts antiproliferative and antioxidant activities in vitro. Antioxidants 2019, 8, 183. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Xing, R.; Liu, S.; Yu, H.; Li, P. Role of Fucoxanthin towards Cadmium-induced renal impairment with the antioxidant and anti-lipid peroxide activities. Bioengineered 2021, 12, 7235–7247. [Google Scholar] [CrossRef]
- Zheng, J.; Tian, X.; Zhang, W.; Zheng, P.; Huang, F.; Ding, G.; Yang, Z. Protective effects of fucoxanthin against alcoholic liver injury by activation of Nrf2-Mediated antioxidant defense and inhibition of TLR4-Mediated Inflammation. Mar. Drugs 2019, 17, 552. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.P.; Tong, Q.Y.; Zheng, S.H.; Zhou, M.D.; Zeng, Y.M.; Zhou, T.T. Anti-inflammatory effect of fucoxanthin on dextran sulfate sodium-induced colitis in mice. Nat. Prod. Res. 2020, 34, 1791–1795. [Google Scholar] [CrossRef]
- Zhao, D.; Kwon, S.H.; Chun, Y.S.; Gu, M.Y.; Yang, H.O. Anti-Neuroinflammatory Effects of Fucoxanthin via Inhibition of Akt/NF-κB and MAPKs/AP-1 Pathways and Activation of PKA/CREB Pathway in Lipopolysaccharide-Activated BV-2 Microglial Cells. Neurochem. Res. 2017, 42, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, Y.; Yang, J.; Jin, L.; Gao, Z.; Xue, L.; Hou, L.; Sui, L.; Liu, J.; Zou, X. Fucoxanthin inhibits tumour-related lymphangiogenesis and growth of breast cancer. J. Cell. Mol. Med. 2019, 23, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Almeida, T.P.; Ferreira, J.; Vettorazzi, A.; Azqueta, A.; Rocha, E.; Ramos, A.A. Cytotoxic activity of fucoxanthin, alone and in combination with the cancer drugs imatinib and doxorubicin, in CML cell lines. Environ. Toxicol. Pharmacol. 2018, 59, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Terasaki, M.; Ikuta, M.; Kojima, H.; Tanaka, T.; Maeda, H.; Miyashita, K.; Mutoh, M. Dietary fucoxanthin induces anoikis in colorectal adenocarcinoma by suppressing integrin signaling in a murine colorectal cancer model. J. Clin. Med. 2020, 9, 90. [Google Scholar] [CrossRef] [Green Version]
- Hitoe, S.; Shimoda, H. Seaweed fucoxanthin supplementation improves obesity parameters in mild obese Japanese subjects. Funct. Foods Health Dis. 2017, 7, 246–262. [Google Scholar] [CrossRef]
- Chang, Y.H.; Chen, Y.L.; Huang, W.C.; Liou, C.J. Fucoxanthin attenuates fatty acid-induced lipid accumulation in FL83B hepatocytes through regulated Sirt1/AMPK signaling pathway. Biochem. Biophys. Res. Commun. 2018, 495, 197–203. [Google Scholar] [CrossRef]
- Sun, X.; Zhao, H.; Liu, Z.; Sun, X.; Zhang, D.; Wang, S.; Xu, Y.; Zhang, G.; Wang, D. Modulation of Gut Microbiota by Fucoxanthin during Alleviation of Obesity in High-Fat Diet-Fed Mice. J. Agric. Food Chem. 2020, 68, 5118–5128. [Google Scholar] [CrossRef]
- Xiang, S.; Liu, F.; Lin, J.; Chen, H.; Huang, C.; Chen, L.; Zhou, Y.; Ye, L.; Zhang, K.; Jin, J.; et al. Fucoxanthin Inhibits β-Amyloid Assembly and Attenuates β-Amyloid Oligomer-Induced Cognitive Impairments. J. Agric. Food Chem. 2017, 65, 4092–4102. [Google Scholar] [CrossRef]
- Mohibbullah, M.; Haque, M.N.; Khan, M.N.A.; Park, I.S.; Moon, I.S.; Hong, Y.K. Neuroprotective effects of fucoxanthin and its derivative fucoxanthinol from the phaeophyte Undaria pinnatifida attenuate oxidative stress in hippocampal neurons. J. Appl. Phycol. 2018, 30, 3243–3252. [Google Scholar] [CrossRef]
- Hudlikar, R.R.; Sargsyan, D.; Li, W.; Wu, R.; Zheng, M.; Kong, A.N. Epigenomic, Transcriptomic, and Protective Effect of Carotenoid Fucoxanthin in High Glucose-Induced Oxidative Stress in Mes13 Kidney Mesangial Cells. Chem. Res. Toxicol. 2021, 34, 713–722. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Zhang, L.; Zhao, G.X.; Chen, Y.; Sun, K.L.; Wang, B. Fucoxanthin attenuates doxorubicin-induced cardiotoxicity via anti-oxidant and anti-apoptotic mechanisms associated with p38, JNK and p53 pathways. J. Funct. Foods 2019, 62. [Google Scholar] [CrossRef]
- Guo, L.; Dang, M.; Song, Q.; Zhang, W.; Li, B. Protective effect of fucoxanthin on ovariectomy-induced osteoporosis in rats. Pharmacogn. Mag. 2020, 16, 242. [Google Scholar]
- Chiang, Y.F.; Chen, H.Y.; Chang, Y.J.; Shih, Y.H.; Shieh, T.M.; Wang, K.L.; Hsia, S.M. Protective effects of fucoxanthin on high glucoseand 4-hydroxynonenal (4-HNE)-induced injury in human retinal pigment epithelial cells. Antioxidants 2020, 9, 1176. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Luna, A.; Ávila-Román, J.; González-Rodríguez, M.L.; Cózar, M.J.; Rabasco, A.M.; Motilva, V.; Talero, E. Fucoxanthin-containing creAm. prevents epidermal hyperplasia and UVB-induced skin erythema in mice. Mar. Drugs 2018, 16, 378. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Lee, D.S.; Park, S.K.; Choi, J.S.; Jung, W.K.; Park, W.S.; Choi, I.W. Fucoxanthin Inhibits Myofibroblast Differentiation and Extracellular Matrix Production in Nasal Polyp-Derived Fibroblasts via Modulation of Smad-Dependent and Smad-Independent Signaling Pathways. Mar. Drugs 2018, 16, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpiński, T.M.; Adamczak, A. Fucoxanthin—An Antibacterial Carotenoid. Antioxidants 2019, 8, 239. [Google Scholar]
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef]
- Kawee-ai, A.; Kuntiya, A.; Kim, S.M. Anticholinesterase and antioxidant activities of fucoxanthin purified from the microalga Phaeodactylum tricornutum. Nat. Prod. Commun. 2013, 8, 1381–1386. [Google Scholar] [CrossRef] [Green Version]
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Haque, M.N.; Moon, I.S. Neuroprotection Against Oxidative Stress: Phytochemicals Targeting TrkB Signaling and the Nrf2-ARE Antioxidant System. Front. Mol. Neurosci. 2020, 13, 116. [Google Scholar] [CrossRef]
- Koduvayur Habeebullah, S.F.; Surendraraj, A.; Jacobsen, C. Isolation of Fucoxanthin from Brown Algae and Its Antioxidant Activity: In Vitro and 5% Fish Oil-In-Water Emulsion. JAOCS J. Am. Oil Chem. Soc. 2018, 95, 835–843. [Google Scholar] [CrossRef]
- Mousavi Nadushan, R.; Hosseinzade, I. Optimization of production and antioxidant activity of fucoxanthin from marine haptophyte algae, Isochrysis galbana. Iran. J. Fish. Sci. 2020, 19, 2901–2908. [Google Scholar] [CrossRef]
- Yang, X.; Guo, G.; Dang, M.; Yan, L.; Kang, X.; Jia, K.; Ren, H. Assessment of the Therapeutic Effects of Fucoxanthin by Attenuating Inflammation in Ovalbumin-Induced Asthma in an Experimental Animal Model. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Lin, T.B.; Peng, H.Y.; Lin, C.H.; Lee, A.S.; Liu, H.J.; Li, C.C.; Tseng, K.W. Protective effects of fucoxanthin dampen pathogen-associated molecular pattern (Pamp) lipopolysaccharide-induced inflammatory action and elevated intraocular pressure by activating nrf2 signaling and generating reactive oxygen species. Antioxidants 2021, 10, 1092. [Google Scholar] [CrossRef] [PubMed]
- Tavares, R.S.N.; Kawakami, C.M.; Pereira, K.C.; Do Amaral, G.T.; Benevenuto, C.G.; Maria-Engler, S.S.; Colepicolo, P.; Debonsi, H.M.; Gaspar, L.R. Fucoxanthin for topical administration, a phototoxic vs. Photoprotective potential in a tiered strategy assessed by in vitro methods. Antioxidants 2020, 9, 328. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.B.; Kang, H.; Li, Y.; Park, Y.K.; Lee, J.Y. Fucoxanthin inhibits lipopolysaccharide-induced inflammation and oxidative stress by activating nuclear factor E2-related factor 2 via the phosphatidylinositol 3-kinase/AKT pathway in macrophages. Eur. J. Nutr. 2021, 60, 3315–3324. [Google Scholar] [CrossRef]
- Li, S.; Ren, X.; Wang, Y.; Hu, J.; Wu, H.; Song, S.; Yan, C. Fucoxanthin alleviates palmitate-induced inflammation in RAW 264.7 cells through improving lipid metabolism and attenuating mitochondrial dysfunction. Food Funct. 2020, 11, 3361–3370. [Google Scholar] [CrossRef]
- Takatani, N.; Kono, Y.; Beppu, F.; Okamatsu-Ogura, Y.; Yamano, Y.; Miyashita, K.; Hosokawa, M. Fucoxanthin inhibits hepatic oxidative stress, inflammation, and fibrosis in diet-induced nonalcoholic steatohepatitis model mice. Biochem. Biophys. Res. Commun. 2020, 528, 305–310. [Google Scholar] [CrossRef]
- Bigagli, E.; D’ambrosio, M.; Cinci, L.; Niccolai, A.; Biondi, N.; Rodolfi, L.; Nascimiento, L.B.D.S.; Tredici, M.R.; Luceri, C. A comparative in vitro evaluation of the anti-inflammatory effects of a tisochrysis lutea extract and fucoxanthin. Mar. Drugs 2021, 19, 334. [Google Scholar] [CrossRef]
- Tavares, R.S.N.; Maria-engler, S.S.; Colepicolo, P.; Debonsi, H.M.; Schäfer-korting, M.; Marx, U.; Gaspar, L.R.; Zoschke, C. Skin irritation testing beyond tissue viability: Fucoxanthin effects on inflammation, homeostasis, and metabolism. Pharmaceutics 2020, 12, 136. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.L.; Jiang, Y.F.; Lu, Y.A.; Yu, J.B.; Kang, M.C.; Jeon, Y.J. Fucoxanthin-rich fraction from Sargassum fusiformis alleviates particulate matter-induced inflammation in vitro and in vivo. Toxicol. Rep. 2021, 8, 349–358. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, G.; Lin, Q.; Tang, Z.; Yan, Q.; Yu, X. Fucoxanthin prevents lipopolysaccharide-induced depressive-like behavior in mice via AMPK- NF-κB pathway. Metab. Brain Dis. 2019, 34, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.J.; Lee, C.J.; Lin, T.B.; Peng, H.Y.; Liu, H.J.; Chen, Y.S.; Tseng, K.W. Protective Effects of Fucoxanthin on Ultraviolet B-Induced Corneal Denervation and Inflammatory Pain in a Rat Model. Mar. Drugs 2019, 17, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.J.; Liou, C.J.; Chen, Y.L.; Cheng, S.C.; Huang, W.C. Fucoxanthin ameliorates oxidative stress and airway inflammation in tracheal epithelial cells and asthmatic mice. Cells 2021, 10, 1311. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Guo, K.; Huang, M.; Liu, Y.; Zhang, J.; Sun, L.; Li, D.; Pang, K.L.; Wang, G.; Chen, L.; et al. Fucoxanthin, a marine xanthophyll isolated from Conticribra weissflogii ND-8: Preventive anti-inflammatory effect in a mouse model of sepsis. Front. Pharmacol. 2019, 10, 906. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.H.; Shin, H.Y.; Park, J.H.; Koo, S.Y.; Kim, S.M.; Yang, S.H. Fucoxanthin from microalgae Phaeodactylum tricornutum inhibits pro-inflammatory cytokines by regulating both NF-κB and NLRP3 inflammasome activation. Sci. Rep. 2021, 11, 543. [Google Scholar] [CrossRef]
- Takatani, N.; Taya, D.; Katsuki, A.; Beppu, F.; Yamano, Y.; Wada, A.; Miyashita, K.; Hosokawa, M. Identification of Paracentrone in Fucoxanthin-Fed Mice and Anti-Inflammatory Effect against Lipopolysaccharide-Stimulated Macrophages and Adipocytes. Mol. Nutr. Food Res. 2021, 65, 405. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Terasaki, M.; Maeda, H.; Miyashita, K.; Tanaka, T.; Miyamoto, S.; Mutoh, M. A marine bio-functional lipid, fucoxanthinol, attenuates human colorectal cancer stem-like cell tumorigenicity and sphere formation. J. Clin. Biochem. Nutr. 2017, 61, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Terasaki, M.; Hamoya, T.; Kubota, A.; Kojima, H.; Tanaka, T.; Maeda, H.; Miyashita, K.; Mutoh, M. Fucoxanthin prevents colorectal cancer development in dextran sodium sulfate-treated ApcMin/+ mice. Anticancer Res. 2021, 41, 1299–1305. [Google Scholar] [CrossRef]
- Terasaki, M.; Ono, S.; Hashimoto, S.; Kubota, A.; Kojima, H.; Ohta, T.; Tanaka, T.; Maeda, H.; Miyashita, K.; Mutoh, M. Suppression of C-C chemokine receptor 1 is a key regulation for colon cancer chemoprevention in AOM/DSS mice by fucoxanthin. J. Nutr. Biochem. 2022, 99, 108871. [Google Scholar] [CrossRef]
- Karkhane Yousefi, M.; Seyed Hashtroudi, M.; Mashinchian Moradi, A.; Ghasempour, A.R. In vitro investigating of anticancer activity of focuxanthin from marine brown seaweed species. Glob. J. Environ. Sci. Manag. 2018, 4, 81–90. [Google Scholar] [CrossRef]
- Lopes, F.G.; Oliveira, K.A.; Lopes, R.G.; Poluceno, G.G.; Simioni, C.; Pescador, G.D.S.; Bauer, C.M.; Maraschin, M.; Derner, R.B.; Garcez, R.C.; et al. Anti-cancer effects of fucoxanthin on human glioblastoma cell line. Anticancer Res. 2020, 40, 6799–6815. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.X.; Yu, R.T.; Liu, Z. Inhibition of two gastric cancer cell lines induced by fucoxanthin involves downregulation of Mcl-1 and STAT3. Hum. Cell 2018, 31, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, H.; Liu, Y. Anti-inflammatory and apoptotic signaling effect of fucoxanthin on benzo(A)pyrene-induced lung cancer in mice. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 239–251. [Google Scholar] [CrossRef]
- Foo, S.C.; Yusoff, F.M.; Imam, M.U.; Foo, J.B.; Ismail, N.; Azmi, N.H.; Tor, Y.S.; Khong, N.M.H.; Ismail, M. Increased fucoxanthin in Chaetoceros calcitrans extract exacerbates apoptosis in liver cancer cells via multiple targeted cellular pathways. Biotechnol. Rep. 2019, 21, e00296. [Google Scholar] [CrossRef]
- Ye, G.; Wang, L.; Yang, K.; Wang, C. Fucoxanthin may inhibit cervical cancer cell proliferation via downregulation of HIST1H3D. J. Int. Med. Res. 2020, 48, 300060520964011. [Google Scholar] [CrossRef]
- Murase, W.; Kamakura, Y.; Kawakami, S.; Yasuda, A.; Wagatsuma, M.; Kubota, A.; Kojima, H.; Ohta, T.; Takahashi, M.; Mutoh, M.; et al. Fucoxanthin prevents pancreatic tumorigenesis in c57bl/6j mice that received allogenic and orthotopic transplants of cancer cells. Int. J. Mol. Sci. 2021, 22, 13620. [Google Scholar] [CrossRef]
- Wang, L.; Wu, R.; Sargsyan, D.; Su, S.; Kuo, H.C.; Li, S.; Chou, P.; Sarwar, M.S.; Phadnis, A.; Wang, Y.; et al. Nfe2l2 Regulates Metabolic Rewiring and Epigenetic Reprogramming in Mediating Cancer Protective Effect by Fucoxanthin. AAPS J. 2022, 24, 30. [Google Scholar] [CrossRef]
- Malhão, F.; Macedo, A.C.; Costa, C.; Rocha, E.; Ramos, A.A. Fucoxanthin holds potential to become a drug adjuvant in breast cancer treatment: Evidence from 2d and 3d cell cultures. Molecules 2021, 26, 4288. [Google Scholar] [CrossRef]
- Ming, J.X.; Wang, Z.C.; Huang, Y.; Ohishi, H.; Wu, R.J.; Shao, Y.; Wang, H.; Qin, M.Y.; Wu, Z.L.; Li, Y.Y.; et al. Fucoxanthin extracted from Laminaria Japonica inhibits metastasis and enhances the sensitivity of lung cancer to Gefitinib. J. Ethnopharmacol. 2021, 265, 113302. [Google Scholar] [CrossRef]
- Gille, A.; Stojnic, B.; Derwenskus, F.; Trautmann, A.; Schmid-Staiger, U.; Posten, C.; Briviba, K.; Palou, A.; Bonet, M.L.; Ribot, J. A lipophilic fucoxanthin-rich Phaeodactylum tricornutum extract ameliorates effects of diet-induced obesity in C57BL/6J. mice. Nutrients 2019, 11, 796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, S.Y.; Hwang, J.H.; Yang, S.H.; Um, J.I.; Hong, K.W.; Kang, K.; Pan, C.H.; Hwang, K.T.; Kim, S.M. Anti-obesity effect of standardized extract of microalga Phaeodactylum tricornutum containing fucoxanthin. Mar. Drugs 2019, 17, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.P.; Baskaran, V. Polysaccharide (laminaran and fucoidan), fucoxanthin and lipids as functional components from brown algae (Padina tetrastromatica) modulates adipogenesis and thermogenesis in diet-induced obesity in C57BL6 mice. Algal. Res. 2021, 54, 102187. [Google Scholar] [CrossRef]
- Guo, B.; Yang, B.; Pang, X.; Chen, T.; Chen, F.; Cheng, K.W. Fucoxanthin modulates cecal and fecal microbiota differently based on diet. Food Funct. 2019, 10, 5644–5655. [Google Scholar] [CrossRef]
- Huang, L.L.; Huang, X.Q.; Zhang, X.Q.; Liu, J.; Zhang, Y.P.; Zhao, H.Y.; Huang, M.Q. Effect of fucoxanthin on insulin resistance in obese mice induced by high fat diet. Zhongguo Zhongyao Zazhi 2021, 46, 171–176. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, W.; Huang, X.; Zhao, Y.; Ren, Q.; Hong, Z.; Huang, M.; Xing, X. Fucoxanthin ameliorates hyperglycemia, hyperlipidemia and insulin resistance in diabetic mice partially through IRS-1/PI3K/Akt and AMPK pathways. J. Funct. Foods 2018, 48, 515–524. [Google Scholar] [CrossRef]
- Yang, G.; Li, Q.; Peng, J.; Jin, L.; Zhu, X.; Zheng, D.; Zhang, Y.; Wang, R.; Song, Y.; Hu, W.; et al. Fucoxanthin regulates Nrf2 signaling to decrease oxidative stress and improves renal fibrosis depending on Sirt1 in HG-induced GMCs and STZ-induced diabetic rats. Eur. J. Pharmacol. 2021, 913, 174629. [Google Scholar] [CrossRef]
- Oliyaei, N.; Moosavi-Nasab, M.; Tamaddon, A.M.; Tanideh, N. Antidiabetic effect of fucoxanthin extracted from Sargassum angustifolium on streptozotocin-nicotinamide-induced type 2 diabetic mice. Food Sci. Nutr. 2021, 9, 3521–3529. [Google Scholar] [CrossRef]
- Wacker-Gussmann, A.; Oberhoffer-Fritz, R. Cardiovascular Risk Factors in Childhood and Adolescence. J. Clin. Med. 2022, 11, 1136. [Google Scholar] [CrossRef]
- Chiang, Y.F.; Tsai, C.H.; Chen, H.Y.; Wang, K.L.; Chang, H.Y.; Huang, Y.J.; Hong, Y.H.; Ali, M.; Shieh, T.M.; Huang, T.C.; et al. Protective Effects of Fucoxanthin on Hydrogen Peroxide-Induced Calcification of Heart Valve Interstitial Cells. Mar. Drugs 2021, 19, 307. [Google Scholar] [CrossRef]
- Rodrigo, R.; González-Montero, J.; Sotomayor, C.G. Novel Combined Antioxidant Strategy against Hypertension, Acute Myocardial Infarction and Postoperative Atrial Fibrillation. Biomedicines 2021, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.M.; Li, K.L.; Lin, Y.C. Fucoidan–fucoxanthin ameliorated cardiac function via IrS1/Grb2/ SOS1, GSK3β/CREB pathways and metabolic pathways in senescent mice. Mar. Drugs 2019, 17, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Zhang, H.; Lv, G.; Liu, Z.; Zheng, X.; Wu, X. Fucoxanthin averts isoprenaline hydrochloride-induced myocardial infarction in rats. Pharmacogn. Mag. 2020, 16, 214. [Google Scholar]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.J.; Kim, S.C.; Lee, J.H.; Lee, J.R.; Kim, I.K.; Baek, S.Y.; Kim, Y.W. Fucoxanthin, the constituent of Laminaria japonica, triggers AMPK-mediated cytoprotection and autophagy in hepatocytes under oxidative stress. BMC Complement. Altern. Med. 2018, 18, 97. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhao, T.; Shi, D.; Ye, M.B.; Yi, Q. Protective role of fucoxanthin in diethylnitrosamine-induced hepatocarcinogenesis in experimental adult rats. Drug Dev. Res. 2019, 80, 209–217. [Google Scholar] [CrossRef]
- Kinsey, G.R.; Okusa, M.D. Pathogenesis of acute kidney injury: Foundation for clinical practice. Am. J. Kidney Dis. 2011, 58, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Lewington, A.J.; Cerda, J.; Mehta, R.L. Raising awareness of acute kidney injury: A global perspective of a silent killer. Kidney Int. 2013, 84, 457–467. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Jin, L.; Zheng, D.; Tang, X.; Yang, J.; Fan, L.; Xie, X. Fucoxanthin alleviates oxidative stress through Akt/SIRT1/FoxO3α signaling to inhibit Hg-induced renal fibrosis in GMCs. Mar. Drugs 2019, 17, 702. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Younis, E.M.; Priya Veeraraghavan, V.; Tian, C. Antiurolithiatic effect of Fucoxanthin on ethylene glycol-induced renal calculus in experimental rats. J. King Saud Univ.-Sci. 2020, 32, 1896–1901. [Google Scholar] [CrossRef]
- Willmann, G. Ultraviolet Keratitis: From the Pathophysiological Basis to Prevention and Clinical Management. High Alt. Med. Biol. 2015, 16, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Dash, R.; Mitra, S.; Ali, M.C.; Oktaviani, D.F.; Hannan, M.A.; Choi, S.M.; Moon, I.S. Phytosterols: Targeting neuroinflammation in neurodegeneration. Curr. Pharm. Des. 2021, 27, 383–401. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Sohag, A.A.M.; Rahman, M.A.; Uddin, M.J.; Alam, M.; Moon, I.S. Neuroprotective Potentials of Marine Algae and Their Bioactive Metabolites: Pharmacological Insights and Therapeutic Advances. Mar. Drugs 2020, 18, 347. [Google Scholar] [CrossRef]
- Lin, J.; Yu, J.; Zhao, J.; Zhang, K.; Zheng, J.; Wang, J.; Huang, C.; Zhang, J.; Yan, X.; Gerwick, W.H.; et al. Fucoxanthin, a Marine Carotenoid, Attenuates β -Amyloid Oligomer-Induced Neurotoxicity Possibly via Regulating the PI3K/Akt and the ERK Pathways in SH-SY5Y Cells. Oxid. Med. Cell. Longev. 2017, 2017, 6792543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Lin, J.J.; Yu, R.; He, S.; Wang, Q.W.; Cui, W.; Zhang, J.R. Fucoxanthin prevents H2O2-induced neuronal apoptosis via concurrently activating the PI3-K/Akt cascade and inhibiting the ERK pathway. Food Nutr. Res. 2017, 61, 1304678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alghazwi, M.; Smid, S.; Musgrave, I.; Zhang, W. In vitro studies of the neuroprotective activities of astaxanthin and fucoxanthin against amyloid beta (Aβ 1-42 ) toxicity and aggregation. Neurochem. Int. 2019, 124, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Dhami, M.; Raj, K.; Singh, S. Neuroprotective Effect of Fucoxanthin against Intracerebroventricular Streptozotocin (ICV-STZ) Induced Cognitive Impairment in Experimental Rats. Curr. Alzheimer Res. 2021, 18, 623–637. [Google Scholar] [CrossRef]
- Yang, M.; Jin, L.; Wu, Z.; Xie, Y.; Zhang, P.; Wang, Q.; Yan, S.; Chen, B.; Liang, H.; Naman, C.B.; et al. PLGA-PEG Nanoparticles Facilitate in Vivo Anti-Alzheimer’s Effects of Fucoxanthin, a Marine Carotenoid Derived from Edible Brown Algae. J. Agric. Food Chem. 2021, 69, 9764–9777. [Google Scholar] [CrossRef]
- Wu, W.; Han, H.; Liu, J.; Tang, M.; Wu, X.; Cao, X.; Zhao, T.; Lu, Y.; Niu, T.; Chen, J.; et al. Fucoxanthin Prevents 6-OHDA-Induced Neurotoxicity by Targeting Keap1. Oxid. Med. Cell. Longev. 2021, 2021, 6688708. [Google Scholar] [CrossRef]
- Hu, L.; Chen, W.; Tian, F.; Yuan, C.; Wang, H.; Yue, H. Neuroprotective role of fucoxanthin against cerebral ischemic/reperfusion injury through activation of Nrf2/HO-1 signaling. Biomed. Pharmacother. 2018, 106, 1484–1489. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Fan, Y.; Gao, Y.; Li, X.; Hu, Z.; Ding, K.; Wang, Y.; Wang, X. Fucoxanthin provides neuroprotection in models of traumatic brain injury via the Nrf2-ARE and Nrf2-autophagy pathways. Sci. Rep. 2017, 7, srep46763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, M.C.; Kim, N.; Kadono, Y.; Rho, J.; Lee, S.Y.; Lorenzo, J.; Choi, Y. Osteoimmunology: Interplay between the immune system and bone metabolism. Annu. Rev. Immunol. 2006, 24, 33–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strait, K.; Li, Y.; Dillehay, D.L.; Weitzmann, M.N. Suppression of NF-κB activation blocks osteoclastic bone resorption during estrogen deficiency. Int. J. Mol. Med. 2008, 21, 521–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ha, Y.J.; Choi, Y.S.; Oh, Y.R.; Kang, E.H.; Khang, G.; Park, Y.B.; Lee, Y.J. Fucoxanthin Suppresses Osteoclastogenesis via Modulation of MAP Kinase and Nrf2 Signaling. Mar. Drugs 2021, 19, 132. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Veeraraghavan, V.P.; Mohan, S.K.; Ma, Y. Restorative Effect of Fucoxanthin in an Ovalbumin-Induced Allergic Rhinitis Animal Model through NF-kappaB p65 and STAT3 Signaling. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 365–375. [Google Scholar] [CrossRef]
- Duncan, F.J.; Martin, J.R.; Wulff, B.C.; Stoner, G.D.; Tober, K.L.; Oberyszyn, T.M.; Kusewitt, D.F.; Van Buskirk, A.M. Topical treatment with black raspberry extract reduces cutaneous UVB-induced carcinogenesis and inflammation. Cancer Prev. Res. 2009, 2, 665–672. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yang, I.; Cao, M.; Su, Z.Y.; Wu, R.; Guo, Y.; Fang, M.; Kong, A.N. Fucoxanthin Elicits Epigenetic Modifications, Nrf2 Activation and Blocking Transformation in Mouse Skin JB6 P+ Cells. AAPS J. 2018, 20, 32. [Google Scholar] [CrossRef]
- Natsume, C.; Aoki, N.; Aoyama, T.; Senda, K.; Matsui, M.; Ikegami, A.; Tanaka, K.; Azuma, Y.T.; Fujita, T. Fucoxanthin Ameliorates Atopic Dermatitis Symptoms by Regulating Keratinocytes and Regulatory Innate Lymphoid Cells. Int. J. Mol. Sci. 2020, 21, 2180. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Sun, X.; Sun, X.; Wang, S.; Xu, Y. Fucoxanthin isolated from Undaria pinnatifida can interact with Escherichia coli and lactobacilli in the intestine and inhibit the growth of pathogenic bacteria. J. Ocean Univ. China 2019, 18, 926–932. [Google Scholar] [CrossRef]
- Chai, L.; Wang, J.; Wei, Y. Fucoxanthin improves functional recovery of orbitopathy in Graves’ disease by downregulating IL-17 mRNA expression in a mouse model. Trop. J. Pharm. Res. 2020, 19, 933–941. [Google Scholar] [CrossRef]
- Yang, H.; Xing, R.; Liu, S.; Li, P. Effect of Fucoxanthin Administration on Thyroid Gland Injury Induced by Cadmium in Mice. Biol. Trace Elem. Res. 2021, 199, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Hosokawa, M.; Miyashita, K.; Nishino, H.; Hashimoto, T. Effects of fucoxanthin on the inhibition of dexamethasone-induced skeletal muscle loss in mice. Nutrients 2021, 13, 1079. [Google Scholar] [CrossRef] [PubMed]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products Towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wu, H.; Wen, H.; Fang, H.; Hong, Z.; Yi, R.; Liu, R. Simultaneous Determination of Fucoxanthin and Its Deacetylated Metabolite Fucoxanthinol in Rat Plasma by Liquid Chromatography-Tandem Mass Spectrometry. Mar. Drugs 2015, 13, 6521–6536. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Ozaki, Y.; Taminato, M.; Das, S.K.; Mizuno, M.; Yoshimura, K.; Maoka, T.; Kanazawa, K. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br. J. Nutr. 2009, 102, 242–248. [Google Scholar] [CrossRef] [Green Version]
- Shikov, A.N.; Flisyuk, E.V.; Obluchinskaya, E.D.; Pozharitskaya, O.N. Pharmacokinetics of Marine-Derived Drugs. Mar. Drugs 2020, 18, 557. [Google Scholar] [CrossRef]
- Beppu, F.; Niwano, Y.; Tsukui, T.; Hosokawa, M.; Miyashita, K. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. J. Toxicol. Sci. 2009, 34, 501–510. [Google Scholar] [CrossRef] [Green Version]
- Kadekaru, T. Safety evaluation of fucoxanthin purified from Undaria pinnatifida. Nippon Shokuhin Kagaku Kogaku Kaishi 2008, 55, 304–308. [Google Scholar] [CrossRef] [Green Version]
- Miyashita, K.; Hosokawa, M.J. Fucoxanthin in the management of obesity and its related disorders. J. Funct. Foods 2017, 36, 195–202. [Google Scholar] [CrossRef]
- Lourenço-Lopes, C.; Garcia-Oliveira, P.; Carpena, M.; Fraga-Corral, M.; Jimenez-Lopez, C.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Scientific approaches on extraction, purification and stability for the commercialization of fucoxanthin recovered from brown algae. Foods 2020, 9, 1113. [Google Scholar] [CrossRef]
- Koo, S.Y.; Mok, I.K.; Pan, C.H.; Kim, S.M. Preparation of Fucoxanthin-Loaded Nanoparticles Composed of Casein and Chitosan with Improved Fucoxanthin Bioavailability. J. Agric. Food Chem. 2016, 64, 9428–9435. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Khalid, N.; Shu, G.; Zhao, Y.; Kobayashi, I.; Neves, M.A.; Tuwo, A.; Nakajima, M. Fucoxanthin-Loaded Oil-in-Water Emulsion-Based Delivery Systems: Effects of Natural Emulsifiers on the Formulation, Stability, and Bioaccessibility. ACS Omega 2019, 4, 10502–10509. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Gu, Y.; Lu, Y.; Yu, C.; Zheng, J.; Qi, H. Fucoxanthin@polyvinylpyrrolidone nanoparticles promoted oxidative stress-induced cell death in Caco-2 human colon cancer cells. Mar. Drugs 2021, 19, 92. [Google Scholar] [CrossRef] [PubMed]




| Experimental Model (In Vitro/In Vivo) | Treatment (Dose, Route and Duration) | Major Outcomes | Reference |
|---|---|---|---|
| Double-blind placebo-controlled study in mild obese Japanese subjects | 1 or 3 mg daily, orally for 4 weeks | ↓ relative body weight and BMI and visceral; ↓ fat area and mass | [15] |
| Fatty acid-induced lipid accumulation in FL83B cells | 3–100 μM (purity ≥ 95%) in DMSO, Post-treatment for 24 h | ↓ lipid accumulation, lipid peroxidation; ↓ PPARγ and SREBP-1c; ↑ CPT-1 and PPAR-a; ↑ Sirt1 and AMPK | [16] |
| Hyperlipidemia in diabetic mice | 0.2–0.4%/day (purity ≥ 98%) extracted from Laminaria japonica, orally for 6 weeks; 0.02% metformin as positive control | ↓ plasma insulin and HOMA-IR; ↓ TG and TC levels; ↓ Glucokinase and phosphoenolpyruvate; Carboxykinase; ↑ Glycogen and GLUT4; ↓ GSK3β; ↑ IRS-1, PI3K, p-Akt and p-AMPK | [66] |
| High-fed diet mice intestine | 125 mg/kg b.w. (purity ≥ 95%) extracted from undaria pinnatifida, orally for 4 weeks | Modulation of gut microbiota to exert anti-obesity effects | [64] |
| HFD-induced obesity mice | 100–300 mg/kg b.w., orally for 26 days | ↑ Cpt1; Ucp1; ↓ Mest; ↓ body weight gain; ↓ fat content; ↓ weight of white adipose tissue depots and size | [61] |
| 3T3-L1 cells | 10–40 μM extracted from Phaeodactylum in DMSO, treatment for 6 days | ↓ lipid accumulation; ↓ C/EBPα, PPARγ and UCP1 | [62] |
| High-fat diet-induced mice | 0.1 mg/kg b.w. extracted from Phaeodactylum in DW water, orally for 6 weeks | ↓ TG level; ↓ lipid droplet numbers and fat globule size ↓ C/EBPα, PPARγ and UCP1 | |
| HFD-fed obese mice | 50–100 mg/100 g diet (purity ≥ 93%) extracted from Undaria pinnatifida, orally for 12 weeks | ↓ body weight gain ↑ HDL-cholesterol level ↓ hepatic steatosis and adipocyte size ↓ IL-6 and TNF-α levels | [17] |
| HFD-induced obese mice | 0.2–0.4% of daily diet, orally for 6 weeks | ↓ body weight, TC, TG, LDL-C and HOMA-IR; ↑ HDL-C; ↑ p-IRS-1, IRS-1, PI3 K and p-Akt | [65] |
| HFD-fed obese mice | 0.5 mg/kg b.w. (purity ≥ 95%) extracted from Padina tetrastromatica, orally for 5 weeks; orlistat 20 mg/kg as positive control | ↓ body weight, TC, TG; ↑ SOD, CAT and GPx; ↑ Akt and UCP-1 ↓ p-Akt, p38 and PPAR-γ | [63] |
| Experimental Model (In Vitro/In Vivo) | Treatment (Dose, Route and Duration) | Major Outcomes | Reference |
|---|---|---|---|
| Type-2 diabetic mice | 0.2–0.4%/day (purity ≥ 98%) extracted from Laminaria japonica in soybean oil, orally for 6 weeks; metformin 0.02% as positive control | ↓ body weight and blood glucose; ↓ plasma insulin, HOMA-IR levels and lipid profile; ↑ Glucokinase mRNA: ↓ phosphoenolpyruvate carboxykinase mRNA; ↑ glycogen synthesis: ↑ IRS-1, PI3K, p-Akt and p-AMPK signaling pathways; ↑ PPARα, p-ACC and CPT-1 protein expression | [66] |
| STZ-and NA-induced diabetic rats | 13–65 mg/kg b.w. extracted from Laminaria japonica, orally for 4 weeks; rosiglitazone 0.571 mg/kg as positive control | ↓ plasma glucose, insulin level and HOMA-IR; ↑ CAT, SOD and GPx; ↓ TNF-α and IL-6; ↑ luteinizing and testosterone hormones | [3] |
| HG-and 4-HNE-induced diabetic retinopathy in ARPE-19 cells | 0.1–0.5 mg/mL, co-treatment for 24–72 h | ↓ cell damage; ↓ inflammation response; ↓ apoptosis; ↓ cell adhesion factor protein; ↓ reactive oxygen species; ↑ antioxidant activity | [23] |
| STZ-and NA-induced type 2 diabetic rats | 400 mg/kg b.w. (purity ≥ 54%) extracted from S. angustifolium, encapsulated with porous starch, orally for 3 weeks; metformin 50 mg/kg as positive control | ↓ weight gain and blood glucose; ↑ plasma insulin; ↓ lipid profile ↑ pancreatic beta cells | [68] |
| HG-induced GMCs in diabetic nephropathy | 2 μM, co-treatment for 24 h | ↓ fibronectin and collagen IV expression; ↑ Sirt1/Nrf2 signaling proteins | [67] |
| STZ-induced diabetic rats | 200 mg/kg b.w., orally for 12 weeks | ↑ renal function and hypertrophy; ↓ glomerulosclerosis; ↓ fibronectin and collagen IV expression; ↑ Sirt1/Nrf2 signaling proteins; ↑ SOD and HO-1; ↓ malondialdehyde level |
| Experimental Model (In Vitro/In Vivo) | Treatment (Dose, Route and Duration) | Major Outcomes | Reference |
|---|---|---|---|
| Aging C57BL mice | 250–500 mg/kg b.w., orally for 28 days | ↓ SOS1 and GRB2; ↓ ROS; ↑ GSK3β, CREB and IRS1 | [72] |
| Doxorubicin-induced cardiac dysfunction mice | 125–500 mg/kg b.w., intraperitoneally for 4 days | ↓ AST, LDH and CKMB | [21] |
| Doxorubicin-treated neonatal rat cardiomyocytes | 50 µM in ddH2O, pre-treatment for 24 h | ↓ ROS; ↓ Bax, p-ERK, p-JNK, p-p38 and p-p53; ↑ GSH and Bcl-2 | |
| Isoprenaline hydrochloride- induced myocardial infarction rats | 50 mg/kg b.w. (purity ≥ 95%), orally for 30 days | ↑ SOD, CAT, GPx and GSH; ↓ CKMB, TNF-α, IL-6 and NF-κB | [73] |
| H2O2-treated rat valve interstitial cells | 0.01–5 mg/mL in ddH2O, pre-treatment for 24 h | ↓ c-PARP, Caspase 3 and Bax; ↑ Bcl-2; ↓ ROS and Akt/p-Akt | [70] |
| Experimental Model (In Vitro/In Vivo) | Treatment (Dose, Route and Duration) | Major Outcomes | Reference |
|---|---|---|---|
| Fatty acid-induced lipid accumulation in FL83B hepatocytes | 3–100 μM (purity ≥ 95%) in DMSO, post-treatment for 24 h | ↓ Sterol regulatory element-binding proteins 1c and peroxisome proliferator-activated receptor γ; ↓ Fatty acid synthase expression, acetyl-CoA carboxylase; ↑ Adipose triglyceride lipase and the phosphorylation of hormone-sensitive lipase, p-AMPK | [16] |
| AA+ iron-induced oxidative stress in HepG2 cells | 30 μM, pretreatment for 1 h | ↑ Autophagic markers (LC3II and beclin-1), AMPK activation; ↓ p-mTOR; ↑ p-ULK1 | [75] |
| DEN-induced liver carcinoma rats | 50 mg/kg b.w., orally for 15 weeks | ↑ Body weight, serum albumin, SOD, CAT, GPx, GR; ↓ ALT, AST, ALP, LDH, GGT, serum bilirubin and stress markers | [76] |
| Alcohol-induced liver injury mice | 10–40 mg/kg b.w. in alcohol, orally for 7 days; silibinin 80 mg/kg b.w. orally as positive control | ↑ T-AOC, GSH-Px, SOD and CAT; ↓ MDA; ↑ ADH and ALDH; ↓ TNF-α, IL-1β, IL-6, IFN -γ; ↑ Nrf2 protein, NQO1, HO-1 and GCLM; ↓ MyD88, p-IκBα and p-NF-κBp65 | [9] |
| Experimental Model (In Vitro/In Vivo) | Treatment (Dose, Route and Duration) | Major Outcomes | Reference |
|---|---|---|---|
| HG-induced renal fibrosis in mesangial cells | 2 µM (purity ≥ 90%), co-treatment for 24 h | ↓ Fibronectin, collagen IV and extracellular matrix; ↓ ROS; ↓ Serine-threonine kinase; ↑ Sirt1, FoxO3α and MnSOD | [79] |
| Ethylene glycol-treated urolithiasis rats | 40–80 mg/kg b.w. (purity ≥ 99%) in potable water, orally for 4 weeks | ↓ AST, ALT, ALP, GGT and LPO; ↑ SOD, CAT, GPx and GSH | [80] |
| HG-treated mesangial kidney Mes13 cells | 1–2 µM (purity ≥ 98%) in 0.1% DMSO, co-treatment for 5 days | ↓ ROS; ↓ Disp2, ATG10 and CYP2E1; ↑ FGF1, WNT7B and Tgfb1i1 | [20] |
| HG-treated glomerular mesangial cells and STZ -induced diabetic rats | N/A | ↑ Sirt1, ↑Nrf2, ↑SOD and ↑HO-1 | [67] |
| Cadmium chloride-treated mice | 10–50 mg/kg b.w., orally for 14 days; shenfukang tablets orally 50 mg/kg b.w./day for 14 days as positive control | ↓ Blood urea nitrogen and KIM-1; ↓ Caspase 3, Caspase 8, Caspase 9, ERK2, NGAL and POD; ↑ SOD, CAT and APX | [8] |
| Experimental model (In Vitro/In Vivo) | Treatment (Dose, Route and Duration) | Major Outcomes | Reference |
|---|---|---|---|
| UVB-Induced corneal denervation rats | 1–10 mg/kg b.w., orally for 6 days | ↑ Nrf2 in cornea; ↓ p38 MAP kinase and GFAP-positive neural cells; ↑ nerve innervation ↓ TRPV1 expression in the trigeminal ganglia neurons; ↓ opening the eyes and eye wipe behavior | [42] |
| High glucose and 4-HNE-induced diabetic retinopathy in ARPE-19 cells | 0.1–0.5 mg/mL, co-treatment for 24–72 h | ↑ cell viability; ↓ DNA damage; ↑ Nrf2 protein; ↓ apoptosis- related protein expression; ↓ ICAM-1; ↑ occludin and ZO-1 protein expressions; ↓ ROS; ↑ antioxidant activity | [23] |
| LPS-induced uveitis rats | 1–10 mg/kg b.w. in 0.1% DMSO, orally for 7 days | ↑ Nrf2 in ocular tissues; ↑ SOD; ↓ MDA; ↓ ocular hypertension; ↓ inflammatory cells and TNF-α; ↓ corneal endothelial disruption | [33] |
| Experimental Model (In Vitro/In Vivo) | Treatment (Dose, Route and Duration) | Major Outcomes | Reference |
|---|---|---|---|
| Ovariectomy-induced osteoporosis rats | 20–40 mg/kg b.w., orally for 16 weeks | ↓ IL-6, TNF-α and IL-1β; ↑ serum levels of E2 and 1,25(OH)2 D3; ↓ RANKL; ↑ OPG levels; ↑ bone mineral contents and density; ↑ normal bone architecture and trabecular formation in femur; | [22] |
| sRANKL and/or NF-κB-induced osteoclast-like RAW264.7 cells | 1–5 μM, pre-treatment for 4 days | ↓ osteoclast differentiation and bone resorption ability; ↓ nuclear factor of activated T cells 1, dendritic cell-specific seven transmembrane protein and matrix metallopeptidase 9; ↓ p38 and ERK; ↑ nuclear translocation of phospho-Nrf2 | [94] |
| Experimental Model (In Vitro/In Vivo) | Treatment (Dose, Route and Duration) | Major Outcomes | Reference |
|---|---|---|---|
| Nasal polyps-derived fibroblast culture | 10–30 µM, treatment for 24 h; TGF-β1 as negative control | ↓ α-SMA and Col-1; ↓ collagen gel contraction; ↓ Smad-2/3 and SP-1 | [25] |
| OVA-induced allergic rhinitis mice | N/A | ↓ ciliary loss, eosinophil infiltration and MDA; ↑ NF-κB p65; ↓ IκBα phosphorylation; ↓ IL-17A expression; ↓ IgE and histamine | [95] |
| OVA-induced asthma mice | 50 mg/kg b.w., oral treatment | ↓ ROS; ↑ antioxidant enzyme activity; ↓inflammatory cytokine markers; | [32] |
| Inflamed tracheal epithelial BEAS-2B cells | 3–30 μM (purity ≥ 95%) in DMSO, pre-treatment for 1 h; TNF-α/IL-4 as negative control | ↓ THP-1 cell adherence; ↓ pro-inflammatory cytokines, eotaxin and ROS | [43] |
| OVA-sensitized mice | 10–30 mg/kg b.w. (purity ≥ 95%) in DMSO, intraperitoneally for every 3 days from day 14 to 27; prednisolone as positive control | ↓ AHR, goblet cell hyperplasia and eosinophil infiltration; ↓ Th2 cytokine production |
| Experimental Model (In Vitro/In Vivo) | Treatment (Dose, Route and Duration) | Major Outcomes | Reference |
|---|---|---|---|
| UVB-irradiated HaCaT cells | 10–100 μM in 0.1% DMSO, pre-treated for 24 h; dexamethasone as positive reference control | ↑ viability; ↓ TNF-α, IL-6; ↓ ROS and LDH production; | [24] |
| TPA-induced epidermal hyperplasia in mice | 200 μg in ethanol of cream formulation/cm2 skin area, topical application for 5 days; β-carotene-cream as positive control | ↓ skin edema, epidermal thickness, MPO activity; ↓ COX-2 and iNOS expression; ↑ HO-1 protein | |
| TPA-induced transformation of JB6 P+ cells | 6.25–50 μM in 0.1% DMSO, co-treatment for 3–24 h; 5-aza-deoxycytidine and trichostatin A as positive control | ↑ Nrf2 and its downstream genes; ↓ colony formation in JB6 P+ cells; ↓ methylation of the Nrf2 promoter region; ↓ DNMT activity | [97] |
| Atopic dermatitis Nc/Nga mice | 0.1% (purity: 70%) in vaseline, topical application for 5 weeks; 0.1% tacrolimus ointment as positive control | ↓ eosinophil infiltration and expression of Il-33; ↑ IL-2, IL-5, IL-13, IL-10 and TGF-β expression; ↑ innate lymphoid cells | [98] |
| Reconstructed human skin in culture plates | 0.5% (w/v) all-trans-fucoxanthin (purity ≥ 95%) in alkyl benzoate or ethanol, pre-treatment for 15 min; sodium dodecyl sulfate as positive control | ↑ viability; ↓IL-6 and IL-8; ↑ NAT1 gene expression | [39] |
| UVA-and UVB-induced 3T3 mouse fibroblast cells and reconstructed human skin | 0.1–100 μg/mL extracted from D. anceps in sunscreen formulation, pre-treatment for 1 h; norfloxacin as positive control | ↓ phototoxicity; ↓ acute photoirritation potential; ↓ ROS | [34] |
| Experimental Model (In Vitro/In Vivo) | Treatment (Dose, Route and Duration) | Major Outcomes | Reference |
|---|---|---|---|
| Agar disc-diffusion | 15.6–1000 μg/mL (purity ≥ 95%) in 20% water solution of DMSO, incubation for 18 h and anaerobes for 2 h | Streptococcus agalactiae (mean ZOI 12.2 mm), Staphylococcus epidermidis (mean ZOI 11.2 mm) and Staphylococcus aureus (mean ZOI 11.0 mm) | [26] |
| Micro-dilution test | 15.6–1000 μg/mL, incubation for 24 h | Streptococcus agalactiae with minimal inhibitory concentration of 62.5 μg/mL | |
| Agar disc-diffusion | 4.25 mg/mL (purity ≥ 82.70%) extracted from Undaria pinnatifida in dehydrated alcohol, incubation for 24 h; chloramphenicol as positive control | ↓ Gram-positive pathogenic bacteria | [99] |
| Gut microbiome of mice cultured in brain heart infusion broth anaerobically | 0.025–0.1 mg/mL, incubation for 48 h | ↑ intestinal beneficial microbes |
| Experimental Model (In Vitro/In Vivo) | Treatment (Dose, Route and Duration) | Major Outcomes | Reference |
|---|---|---|---|
| LPS-induced behavioral defects mice | 50–200 mg/kg b.w. (purity ≥ 95.0%) in 0.5% sodium carboxymethylcellulose, orally for 7 days | ↓ immobility time in forced swimming and tail suspension test; ↓ IL-1β, IL-6 and TNF-α; ↓ iNOS and COX-2 | [41] |
| DSS-induced colitis mice | 50–100 mg/kg b.w., orally for 7 days | ↓ body weight loss; ↓ increase of disease activity index and colon shortening; ↓ colon histological damages; ↓ colonic PGE2, COX-2 and NF-κB levels | [10] |
| Graves’ orbitopathy-induced mice | 50 mg/kg b.w., orally for 4 weeks | ↓ mRNA expression of IL-17 ↓ 8-OHdG and MDA | [100] |
| CdCl2-induced thyroid damage mice | 10–50 mg/kg b.w., orally for 14 days; thyroid tablets 50 mg/kg b.w. as positive control | ↑ T4, T3, catalase and APX levels; ↓ MDA; ↑ apoptosis inhibition; ↓ endoplasmic reticulum stress | [101] |
| Dexamethasone-induced skeletal muscle loss mice | 0.2% of daily diet, orally for 14 days | ↓ muscle atrophy, visceral fat mass and muscle lipid peroxidation; ↑ phosphorylation of mTOR; ↓ activation of AMPK | [102] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohibbullah, M.; Haque, M.N.; Sohag, A.A.M.; Hossain, M.T.; Zahan, M.S.; Uddin, M.J.; Hannan, M.A.; Moon, I.S.; Choi, J.-S. A Systematic Review on Marine Algae-Derived Fucoxanthin: An Update of Pharmacological Insights. Mar. Drugs 2022, 20, 279. https://doi.org/10.3390/md20050279
Mohibbullah M, Haque MN, Sohag AAM, Hossain MT, Zahan MS, Uddin MJ, Hannan MA, Moon IS, Choi J-S. A Systematic Review on Marine Algae-Derived Fucoxanthin: An Update of Pharmacological Insights. Marine Drugs. 2022; 20(5):279. https://doi.org/10.3390/md20050279
Chicago/Turabian StyleMohibbullah, Md., Md. Nazmul Haque, Abdullah Al Mamun Sohag, Md. Tahmeed Hossain, Md. Sarwar Zahan, Md. Jamal Uddin, Md. Abdul Hannan, Il Soo Moon, and Jae-Suk Choi. 2022. "A Systematic Review on Marine Algae-Derived Fucoxanthin: An Update of Pharmacological Insights" Marine Drugs 20, no. 5: 279. https://doi.org/10.3390/md20050279
APA StyleMohibbullah, M., Haque, M. N., Sohag, A. A. M., Hossain, M. T., Zahan, M. S., Uddin, M. J., Hannan, M. A., Moon, I. S., & Choi, J.-S. (2022). A Systematic Review on Marine Algae-Derived Fucoxanthin: An Update of Pharmacological Insights. Marine Drugs, 20(5), 279. https://doi.org/10.3390/md20050279