Nitrogen Sources Affect the Long-Chain Polyunsaturated Fatty Acids Content in Thraustochytrium sp. RT2316-16
Abstract
:1. Introduction
2. Results
2.1. Effect of the Organic Nitrogen Source on Production of Biomass and Lipids by RT2316-16
2.2. Effect of the Initial Concentrations of Glucose and Organic Nitrogen Source
2.3. Effect of Other Carbon Sources
2.4. Composition of the Total Lipids in RT2316-16 during the First 48 h of Incubation
2.5. Composition of the Fatty Acids in the Lipids of the Biomass Grown in Different Media
3. Discussion
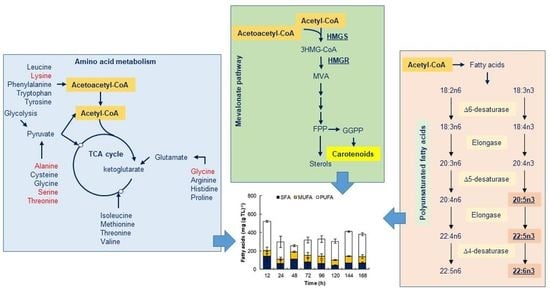
3.1. Metabolism of Yeast Extract Nitrogen and Amino Acids in RT2316-16
3.2. Organic Nitrogen Promoted Mobilization of the Accumulated Lipids in RT2316-16
3.3. Carotenoid Synthesis and Possible Functions in RT2316-16
3.4. EPA, DPA and DHA Accumulated during Consumption of the Accumulated Fatty Acids in RT2316-16
4. Materials and Methods
4.1. Culture Experiments
4.2. Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Suzuki, H.; Manabe, S.; Wada, O.; Crawford, M.A. Rapid incorporation of docosahexaenoic acid from dietary sources into brain microsomal, synaptosomal and mitochondrial membranes in adult mice. Int. J. Vitam. Nutr. Res. 1997, 67, 272–278. [Google Scholar] [PubMed]
- Uauy, R.; Dangour, A.D. Nutrition in brain development and aging: Role of essential fatty acids. Nutr. Rev. 2006, 64, S24–S33. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Cameron-Smith, D.; Garg, M.; Sinclair, A.J. Docosapentaenoic acid (22:5n-3): A review of its biological effects. Prog. Lipid Res. 2011, 50, 28–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishibashi, Y.; Goda, H.; Hamaguchi, R.; Sakaguchi, K.; Sekiguchi, T.; Ishiwata, Y.; Okita, Y.; Mochinaga, S.; Ikeuchi, S.; Mizobuchi, T.; et al. PUFA synthase-independent DHA synthesis pathway in Parietichytrium sp. and its modification to produce EPA and n-3DPA. Commun. Biol. 2021, 4, 1378. [Google Scholar] [CrossRef]
- Zhu, X.; Li, S.; Liu, L.; Li, S.; Luo, Y.; Lv, C.; Wang, B.; Cheng, C.H.K.; Chen, H.; Yang, X. Genome sequencing and analysis of Thraustochytriidae sp. SZU445 provides novel insights into the polyunsaturated fatty acid biosynthesis pathway. Mar. Drugs 2020, 18, 118. [Google Scholar] [CrossRef] [Green Version]
- Leyton, A.; Shene, C.; Chisti, Y.; Asenjo, J.A. Production of carotenoids and phospholipids by Thraustochytrium sp. in batch and repeated-batch culture. Mar. Drugs 2022, 20, 416. [Google Scholar] [CrossRef]
- Morabito, C.; Bournaud, C.; Maës, C.; Schuler, M.; Aiese Cigliano, R.; Dellero, Y.; Maréchal, E.; Amato, A.; Rébeillé, F. The lipid metabolism in thraustochytrids. Prog. Lipid Res. 2019, 76, 101007. [Google Scholar] [CrossRef]
- Auma, K.; Hamid, A.; Yusoff, W. Effect of nitrogen sources on biomass, lipid and docosahexanoic acid production by Aurantiochytrium sp. SW1. AIP Conf. Proc. 2018, 1940, 020065. [Google Scholar] [CrossRef]
- Shene, C.; Leyton, A.; Rubilar, M.; Pinelo, M.; Acevedo, F.; Morales, E. Production of lipids and docosahexaenoic acid (DHA) by a native Thraustochytrium strain. Eur. J. Lipid Sci. Technol. 2013, 115, 890–900. [Google Scholar] [CrossRef]
- Paredes, P.; Larama, G.; Flores, L.; Leyton, A.; Ili, C.G.; Asenjo, J.A.; Chisti, Y.; Shene, C. Temperature differentially affects gene expression in Antarctic thraustochytrid Oblongichytrium sp. RT2316-13. Mar. Drugs 2020, 18, 563. [Google Scholar] [CrossRef]
- Svedas, V.J.; Galaev, I.J.; Borisov, I.L.; Berezin, I.V. The interaction of amino acids with ophthaldialdehyde: A kinetic study and spectrophotometric assay of the reaction product. Anal. Biochem. 1980, 101, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Posch, A.E.; Spadiut, O.; Herwig, C. Switching industrial production processes from complex to defined media: Method development and case study using the example of Penicillium chrysogenum. Microb. Cell Fact. 2012, 11, 88. [Google Scholar] [CrossRef] [Green Version]
- Schwechheimer, S.K.; Becker, J.; Peyriga, L.; Portais, J.C.; Wittmann, C. Metabolic flux analysis in Ashbya gossypii using 13C-labeled yeast extract: Industrial riboflavin production under complex nutrient conditions. Microb. Cell Fact. 2018, 17, 162. [Google Scholar] [CrossRef] [Green Version]
- Jakobsen, A.N.; Aasen, I.M.; Josefsen, K.D.; Strøm, A.R. Accumulation of docosahexaenoic acid-rich lipid in thraustochytrid Aurantiochytrium sp. strain t66: Effects of N and P starvation and O2 limitation. Appl. Microbiol. Biotechnol. 2008, 80, 297. [Google Scholar] [CrossRef] [PubMed]
- Hensley, C.T.; Wasti, A.T.; DeBerardinis, R.J. Glutamine and cancer: Cell biology, physiology, and clinical opportunities. J. Clin. Investig. 2013, 123, 3678–3684. [Google Scholar] [CrossRef] [Green Version]
- Leyton, A.; Flores, L.; Shene, C.; Chisti, Y.; Larama, G.; Asenjo, J.A.; Armenta, R.E. Antarctic thraustochytrids as sources of carotenoids and high-value fatty acids. Mar. Drugs 2021, 19, 386. [Google Scholar] [CrossRef] [PubMed]
- Dellero, Y.; Maës, C.; Morabito, C.; Schuler, M.; Bournaud, C.C.; Cigliano, R.A.; Maréchal, E.; Amato, A.; Rébeillé, F. The zoospores of the thraustochytrid Aurantiochytrium limacinum: Transcriptional reprogramming and lipid metabolism associated to their specific functions. Environ. Microbiol. 2020, 22, 1901–1916. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. Regulation of the mevalonate pathway. Nature 1990, 343, 425–430. [Google Scholar] [CrossRef]
- Goldstein, J.L.; DeBose-Boyd, R.A.; Brown, M.S. Protein sensors for membrane sterols. Cell 2006, 124, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Burg, J.S.; Espenshade, P.J. Regulation of HMG-CoA reductase in mammals and yeast. Prog. Lipid Res. 2011, 50, 403–410. [Google Scholar] [CrossRef]
- Wanders, R.J.A.; Vreken, P.; Ferdinandusse, S.; Jansen, G.A.; Waterham, H.R.; van Roermund, C.W.T.; Van Grunsven, E.G. Peroxisomal fatty acid α- and β-oxidation in humans: Enzymology, peroxisomal metabolite transporters and peroxisomal diseases. Biochem. Soc. Trans. 2001, 29, 250–267. [Google Scholar] [CrossRef] [PubMed]
- Speijer, D. Oxygen radicals shaping evolution: Why fatty acid catabolism leads to peroxisomes while neurons do without it: FADH2/NADH flux ratios determining mitochondrial radical formation were crucial for the eukaryotic invention of peroxisomes and catabolic tissue. BioEssays 2011, 33, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Mercader, J.; Madsen, L.; Felipe, F.; Palou, A.; Kristiansen, K.; Bonet, M.L. All-trans retinoic acid increases oxidative metabolism in mature adipocytes. Cell Physiol. Biochem. 2007, 20, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Chambon, P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996, 10, 940–954. [Google Scholar] [CrossRef]
- Boulanger, A.; McLemore, P.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Yu, S.S.; Gentleman, S.; Redmond, T.M. Identification of β-carotene 15, 15′-monooxygenase as a peroxisome proliferator-activated receptor target gene. FASEB J. 2003, 17, 1304–1306. [Google Scholar] [CrossRef]
- Liu, L.; Wang, F.; Yang, J.; Li, X.; Cui, J.; Liu, J.; Shi, M.; Wang, K.; Chen, L.; Zhang, W. Nitrogen feeding strategies and metabolomic analysis to alleviate high-nitrogen inhibition on docosahexaenoic acid production in Crypthecodinium cohnii. J. Agric. Food Chem. 2018, 66, 10640–10650. [Google Scholar] [CrossRef]
- Clarke, S.D. Nonalcoholic steatosis and steatohepatitis. I. Molecular mechanism for polyunsaturated fatty acid regulation of gene transcription. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G865–G869. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Qiu, X. Analysis of the biosynthetic process of fatty acids in Thraustochytrium. Biochimie 2018, 144, 108–114. [Google Scholar] [CrossRef]
- Bligh, E.; Dyer, W. A rapid method for total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Kishimoto, K.; Urade, R.; Ogawa, T.; Moriyama, T. Nondestructive quantification of neutral lipids by thin-layer chromatography and laser-fluorescent scanning: Suitable methods for “Lipidome” analysis. Biochem. Biophys. Res. Commun. 2001, 281, 657–662. [Google Scholar] [CrossRef]









| Growth Medium * | C-Source | N-Source | Xlf | TC | TL | ||||
|---|---|---|---|---|---|---|---|---|---|
| −rS | R2 | −rAA×10 | R2 | rXlf | rTC | R2 | rTL | R2 | |
| M | 0.17 | 0.978 | 0.78 | 0.955 | 0.09 | 1.30 | 0.953 | 4.07 | 0.966 |
| 0.06 | 0.942 | 1.22 | 0.963 | ||||||
| ML | 0.08 | 0.960 | 0.84 | 0.944 | 0.12 | 1.25 | 0.961 | 3.16 | 0.983 |
| 0.29 | 0.967 | −0.87 | 0.945 | −1.15 | 0.922 | ||||
| MH | 0.13 | 0.989 | 1.55 | 0.999 | 0.20 | 2.77 | 1.000 | 4.04 | 1.000 |
| 0.31 | 0.979 | 0.42 | 0.904 | 0.45 | 0.777 | ||||
| M-CO | n.d. | n.d. | 0.94 | 0.797 | 0.07 | 1.22 | 0.960 | 0.0 | |
| 0.16 | 0.967 | 5.24 | 0.995 | ||||||
| M-Glycerol | 0.08 | 0.977 | 0.68 | 0.96 | 0.07 | 1.84 | 0.757 | 5.00 | 0.962 |
| 0.02 | 0.996 | 0.05 | 0.0 | 13.08 | 0.995 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdebenito, D.; Urrutia, S.; Leyton, A.; Chisti, Y.; Asenjo, J.A.; Shene, C. Nitrogen Sources Affect the Long-Chain Polyunsaturated Fatty Acids Content in Thraustochytrium sp. RT2316-16. Mar. Drugs 2023, 21, 15. https://doi.org/10.3390/md21010015
Valdebenito D, Urrutia S, Leyton A, Chisti Y, Asenjo JA, Shene C. Nitrogen Sources Affect the Long-Chain Polyunsaturated Fatty Acids Content in Thraustochytrium sp. RT2316-16. Marine Drugs. 2023; 21(1):15. https://doi.org/10.3390/md21010015
Chicago/Turabian StyleValdebenito, Diego, Sebastián Urrutia, Allison Leyton, Yusuf Chisti, Juan A. Asenjo, and Carolina Shene. 2023. "Nitrogen Sources Affect the Long-Chain Polyunsaturated Fatty Acids Content in Thraustochytrium sp. RT2316-16" Marine Drugs 21, no. 1: 15. https://doi.org/10.3390/md21010015
APA StyleValdebenito, D., Urrutia, S., Leyton, A., Chisti, Y., Asenjo, J. A., & Shene, C. (2023). Nitrogen Sources Affect the Long-Chain Polyunsaturated Fatty Acids Content in Thraustochytrium sp. RT2316-16. Marine Drugs, 21(1), 15. https://doi.org/10.3390/md21010015