Variability in Macro- and Micronutrients of 15 Rarely Researched Microalgae
Abstract
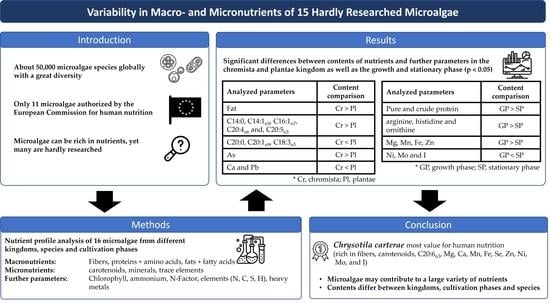
:1. Introduction
2. Results
2.1. Amino Acid Analysis
2.2. Nitrogenous Compounds
| Species | Chrysotila carterae | Eustigmatos sp. | Microchloropsis salina | Nannochloropsis limnetica | Nitzschia palea | Phaeodactylum tricornutum | Autumnella lusatica | Botryococcus braunii | Chlorococcum novae-angliae | Klebsormidium sp. | Myrmecia bisecta | Spongiochloris minor | Stichococcus sp. | Tetradesmus obliquus | Tetraselmis suecica | Chlorococcum novae-angliae | Microchloropsis salina | Tetradesmus obliquus | Spongiochloris minor | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kingdom | Cr | Pl | Pl | Cr | Pl | Pl | |||||||||||||||
| CP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | ◊ | GP | GP | GP | GP | O |
| Alanine | 1.89 | 1.26 | 0.77 ± 0.15 | 1.32 | 1.90 ± 0.04 | 1.28 | 1.95 | 1.06 ± 0.07 | 1.43 ± 0.02 | 1.97 | 2.66 | 2.52 ± 0.14 | 1.156 ± 0.002 | 1.71 ± 0.05 | 0.80 | 0.38 | 2.92 ± 0.37 | 2.56 ± 0.14 | 1.71 ± 0.05 | 2.03 ± 0.10 | 0.12 |
| Arginine | 1.34 | 0.97 | 0.57 ± 0.10 | 1.00 | 1.25 ± 0.03 | 0.89 | 1.54 | 1.14 ± 0.07 | 0.92 ± 0.01 | 1.37 | 1.32 | 3.18 ± 0.18 | 0.81 ± 0.01 | 0.88 ± 0.02 | 0.53 | 0.54 | 2.14 ± 0.26 | 2.03 ± 0.14 | 3.03 ± 0.08 | 1.27 ± 0.04 | 0.04 |
| Aspartic acid | 2.20 | 1.62 | 0.99 ± 0.17 | 1.58 | 2.65 ± 0.04 | 1.78 | 2.24 | 1.35 ± 0.08 | 1.77 ± 0.02 | 2.49 | 2.63 | 3.09 ± 0.21 | 1.22 ± 0.01 | 1.72 ± 0.05 | 0.99 | 0.73 | 3.59 ± 0.50 | 3.43 ± 0.03 | 1.94 ± 0.07 | 1.84 ± 0.18 | 0.16 |
| Cysteine | 0.04 | 0.02 | 0.029 ± 0.004 | 0.03 | 0.039 ± 0.003 | 0.01 | 0.01 | 0.06 ± 0.03 | 0.114 ± 0.001 | 0.01 | 0.04 | 0.12 ± 0.05 | 0.021 ± 0.001 | 0.081 ± 0.001 | <LOQ | 0.25 | 0.26 ± 0.05 | 0.06 ± 0.01 | 0.08 ± 0.0.1 | 0.06 ± 0.01 | 0.78 |
| Glutamic acid | 2.95 | 1.93 | 1.00 ± 0.25 | 2.04 | 2.63 ± 0.05 | 2.08 | 2.69 | 1.58 ± 0.10 | 1.74 ± 0.01 | 3.37 | 3.01 | 3.26 ± 0.19 | 1.44 ± 0.01 | 2.05 ± 0.04 | 1.20 | 0.79 | 3.61 ± 0.39 | 3.37 ± 0.03 | 2.11 ± 0.01 | 2.31 ± 0.12 | 0.12 |
| Glycine | 1.35 | 1.05 | 0.63 ± 0.12 | 1.04 | 1.77 ± 0.03 | 1.01 | 1.55 | 0.86 ± 0.05 | 1.03 ± 0.01 | 1.57 | 1.75 | 1.80 ± 0.10 | 0.83 ± 0.01 | 1.19 ± 0.05 | 0.62 | 0.84 | 2.14 ± 0.28 | 2.01 ± 0.12 | 1.24 ± 0.04 | 1.47 ± 0.06 | 0.12 |
| Histidine | 0.38 | 0.31 | 0.28 ± 0.06 | 0.08 | 0.40 ± 0.01 | 0.07 | 0.51 | 0.28 ± 0.02 | 0.29 ± 0. | 0.49 | 0.58 | 0.42 ± 0.06 | 0.054 ± 0.001 | 0.30 ± 0.01 | 0.19 | 0.89 | 0.69 ± 0.07 | 0.81 ± 0.13 | 0.42 ± 0.04 | 0.36 ± 0.02 | 0.02 |
| Isoleucine | 1.02 | 0.73 | 0.50 ± 0.08 | 0.72 | 1.08 ± 0.02 | 0.78 | 0.94 | 0.64 ± 0.04 | 0.79 ± 0.02 | 0.95 | 1.04 | 1.21 ± 0.07 | 0.516 ± 0.003 | 0.78 ± 0.03 | 0.44 | 0.95 | 1.56 ± 0.19 | 1.62 ± 0.13 | 0.80 ± 0.03 | 0.93 ± 0.01 | 0.12 |
| Leucine | 2.11 | 1.40 | 0.93 ± 0.16 | 1.53 | 1.91 ± 0.03 | 1.41 | 2.19 | 1.38 ± 0.09 | 1.63 ± 0.02 | 2.18 | 2.31 | 2.48 ± 0.17 | 1.14 ± 0.01 | 1.62 ± 0.05 | 0.89 | 0.31 | 3.21 ± 0.41 | 3.06 ± 0.22 | 1.65 ± 0.05 | 2.02 ± 0.07 | 0.12 |
| Lysine | 1.24 | 1.14 | 0.56 ± 0.10 | 1.15 | 1.21 ± 0.02 | 1.02 | 1.55 | 0.89 ± 0.06 | 1.07 ± 0.01 | 1.43 | 1.37 | 1.81 ± 0.07 | 0.907 ± 0.001 | 1.03 ± 0.05 | 0.67 | 0.79 | 2.19 ± 0.26 | 1.76 ± 0.10 | 1.29 ± 0.07 | 1.28 ± 0.01 | 0.12 |
| Methionine | 0.45 | 0.10 | 0.03 ± 0.01 | 0.17 | 0.18 ± 0.08 | 0.32 | 0.33 | 0.07 ± 0.02 | 0.04 ± 0.01 | 0.36 | 0.18 | 0.26 ± 0.07 | 0.10 ± 0.02 | 0.02 ± 0.02 | 0.32 | 0.79 | 0.07 ± 0.01 | 0.19 ± 0.05 | 0.086 ± 0.003 | 0.038 ± 0.001 | 0.91 |
| Phenylalanine | 1.34 | 0.84 | 0.66 ± 0.06 | 0.89 | 1.36 ± 0.03 | 1.09 | 1.52 | 0.86 ± 0.06 | 1.08 ± 0.01 | 1.46 | 1.54 | 1.57 ± 0.11 | 0.67 ± 0.01 | 1.06 ± 0.03 | 0.58 | 0.59 | 2.13 ± 0.23 | 1.90 ± 0.14 | 1.04 ± 0.03 | 1.37 ± 0.05 | 0.12 |
| Proline | 1.15 | 0.82 | 0.55 ± 0.04 | 0.78 | 1.15 ± 0.01 | 0.67 | 1.34 | 0.79 ± 0.03 | 1.26 ± 0.30 | 1.47 | 1.43 | 1.50 ± 0.09 | 0.95 ± 0.23 | 1.27 ± 0.28 | 0.49 | 0.05 | 2.08 ± 0.47 | 1.93 ± 0.40 | 1.32 ± 0.29 | 1.21 ± 0.09 | 0.26 |
| Serine | 1.22 | 0.78 | 0.48 ± 0.10 | 0.86 | 1.48 ± 0.03 | 1.00 | 1.24 | 0.64 ± 0.04 | 0.92 ± 0.01 | 1.29 | 1.16 | 1.45 ± 0.10 | 0.68 ± 0.01 | 0.88 ± 0.01 | 0.56 | 0.99 | 1.81 ± 0.19 | 1.51 ± 0.07 | 0.917 ± 0.003 | 1.04 ± 0.07 | 0.16 |
| Threonine | 1.18 | 0.90 | 0.58 ± 0.11 | 0.83 | 1.36 ± 0.03 | 0.89 | 1.19 | 0.75 ± 0.05 | 1.000 ± 0.001 | 1.35 | 1.32 | 1.71 ± 0.11 | 0.80 ± 0.01 | 1.11 ± 0.02 | 0.57 | 0.59 | 1.95 ± 0.23 | 1.77 ± 0.10 | 1.18 ± 0.03 | 1.26 ± 0.06 | 0.12 |
| Tryptophan | 0.18 | 0.09 | 0.101 ± 0.004 | <LOQ | 0.14 ± 0.01 | 0.04 | 0.27 | 0.075 ± 0.001 | 0.089 ± 0.007 | 0.32 | 0.04 | 0.16 ± 0.02 | 0.022 ± 0.02 | 0.084 ± 0.004 | 0.03 | 0.54 | 0.16 ± 0.03 | 0.20 ± 0.05 | 0.115 ± 0.002 | 0.11 ± 0.01 | 0.12 |
| Tyrosine | 0.66 | 0.57 | 0.25 ± 0.03 | 0.64 | 1.11 ± 0.03 | 0.72 | 1.02 | 0.49 ± 0.01 | 0.15 ± 0.02 | 1.08 | 1.02 | 0.67 ± 0.06 | 0.50 ± 0.01 | 0.20 ± 0.01 | 0.38 | 0.22 | 0.69 ± 0.01 | 0.83 ± 0.02 | 0.41 ± 0.02 | 0.43 ± 0.04 | 0.09 |
| Valine | 1.35 | 0.98 | 0.65 ± 0.10 | 0.96 | 1.31 ± 0.03 | 0.96 | 1.39 | 0.84 ± 0.06 | 1.14 ± 0.02 | 1.32 | 1.45 | 1.85 ± 0.10 | 0.721 ± 0.005 | 1.17 ± 0.03 | 0.65 | 0.54 | 2.19 ± 0.28 | 2.02 ± 0.16 | 1.20 ± 0.05 | 1.31 ± 0.02 | 0.16 |
| Taurine | 0.13 | 0.18 | 0.05 ± 0.01 | 0.08 | 0.039 ± 0.001 | 0.12 | 0.07 | 0.019 ± 0.003 | 0.19 ± 0.01 | 0.09 | 0.08 | 0.202 ± 0.002 | 0.106 ± 0.002 | 0.14 ± 0.04 | 0.20 | 0.17 | 0.09 ± 0.02 | 0.054 ± 0.001 | 0.194 ± 0.005 | 0.079 ± 0.003 | 0.16 |
| S-adenosyl-methionine | <LOQ | <LOQ | 0.01 ± 0.01 | 0.06 | 0.018 ± 0.005 | 0.05 | 0.06 | <LOQ | 0.07 ± 0.01 | 0.01 | 0.08 | 0.07 ± 0.02 | 0.05 ± 0.05 | 0.029 ± 0.003 | <LOQ | 0.17 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.01 ± 0.01 | <LOQ | 0.09 |
| Cystine | 0.40 | 0.17 | 0.08 ± 0.01 | 0.18 | 0.197 ± 0.001 | 0.12 | 0.16 | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.19 | 0.29 | 0.21 ± 0.04 | 0.127 ± 0.006 | 0.10 ± 0.01 | 0.12 | 0.45 | 0.16 ± 0.02 | 0.16 ± 0.06 | 0.13 ± 0.01 | 0.11 ± 0.02 | 0.67 |
| γ-amino-butyric acid | 0.03 | <LOQ | 0.13 ± 0.03 | <LOQ | 0.037 ± 0.001 | 0.02 | 0.47 | 0.024 ± 0.005 | 0.03 ± 0.01 | 0.03 | 0.03 | 0.08 ± 0.03 | <LOQ. | 0.500 ± 0.001 | 0.01 | 0.71 | 0.062 ± 0.002 | 0.23 ± 0.02 | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.67 |
| Ornithine | 0.04 | 0.02 | 0.02 ± 0.01 | 0.03 | 0.151 ± 0.001 | 0.04 | 0.05 | 0.016 ± 0.002 | 0.033 ± 0.003 | 0.02 | 0.09 | 0.061 ± 0.001 | 0.013 ± 0.001 | 0.029 ± 0.002 | <LOQ | 0.38 | 0.07 ± 0.01 | 0.12 ± 0.01 | 0.08 ± 0.01 | 0.027 ± 0.001 | 0.03 |
| NH4+ | 0.41 | 0.36 | 0.23 ± 0.03 | 0.35 | 0.52 ± 0.01 | 0.00 | 0.00 | 0.26 ± 0.03 | 0.49 ± 0.03 | 0.39 | 0.54 | 0.73 ± 0.01 | 0.275 ± 0.001 | 0.41 ± 0.02 | 0.20 | 0.52 | 0.88 ± 0.07 | 0.69 ± 0.06 | 0.51 ± 0.04 | 0.52 ± 0.02 | 0.26 |
| Sum | |||||||||||||||||||||
| NEA | 11.46 | 8.05 | 4.70 ± 0.65 | 8.27 | 12.72 ± 0.28 | 8.56 | 12.04 | 6.83 ± 0.39 | 8.42 ± 0.35 | 13.25 | 13.71 | 14.41 ± 0.64 | 6.80 ±0.24 | 9.10 ± 0.42 | 5.03 | 0.48 | 17.10 ± 1.22 | 15.71 ± 0.68 | 9.74 ± 0.43 | 10.39 ± 0.51 | 0.27 |
| SEA | 1.72 | 1.27 | 0.86 ± 0.11 | 1.08 | 1.65 ± 0.03 | 0.97 | 2.05 | 1.42 ± 0.08 | 1.21 ± 0.01 | 1.85 | 1.90 | 3.60 ± 0.19 | 0.87 ± 0.01 | 1.18 ±0.02 | 0.71 | 0.35 | 2.83 ± 0.27 | 2.84 ±0.19 | 3.46 ± 0.09 | 1.63 ± 0.04 | 0.14 |
| EAA | 8.89 | 6.20 | 4.11 ± 0.26 | 6.25 | 8.54 ± 0.11 | 6.52 | 9.38 | 5.51 ± 0.15 | 6.84 ± 0.04 | 9.36 | 9.25 | 11.05 ± 0.28 | 4.87 ± 0.02 | 6.89 0.09 | 4.15 | 0.35 | 13.46 ± 0.68 | 12.52 ± 0.37 | 7.36 ± 0.12 | 8.32 ± 0.11 | 0.27 |
| N-factor | 4.83 | 5.46 | 4.38 | 4.67 | 4.66 | 5.47 | 5.14 | 4.56 | 4.42 | 4.76 | 4.89 | 5.61 | 4.84 | 4.65 | 4.81 | 0.99 | 4.77 | 4.77 | 4.24 | 3.93 | 0.47 |
| Species | Chrysotila carterae | Eustigmatos sp. | Microchloropsis salina | Nannochloropsis limnetica | Nitzschia palea | Phaeodactylum tricornutum | Autumnella lusatica | Botryococcus braunii | Chlorococcum novae-angliae | Klebsormidium sp. | Myrmecia bisecta | Spongiochloris minor | Stichococcus sp. | Tetradesmus obliquus | Tetraselmis suecica | Chlorococcum novae-angliae | Microchloropsis salina | Tetradesmus obliquus | Spongiochloris minor | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kingdom | Cr | Pl | Pl | Cr | Pl | Pl | |||||||||||||||
| CP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | ◊ | GP | GP | GP | GP | O |
| Nitrogen | 4.71 ± 0.06 | 2.41 ± 0.07 | 2.44 ± 0.01 | 3.49 ± 0.01 | 5.14 ± 0.03 | 3.05 ± 0.01 | 3.91 ± 0.05 | 3.20 ± 0.02 | 3.91 ± 0.05 | 5.56 ± 0.07 | 4.96 ± 0.06 | 4.05 ± 0.03 | 2.55 ± 0.01 | 3.77 ± 0.07 | 2.12 ± 0.01 | 0.41 | 6.72 ± 0.05 | 6.44 ± 0.02 | 6.79 ± 0.10 | 4.83 ± 0.02 | 0.29 |
| NPN | 0.83 ± 0.06 | 0.15 ± 0.08 | 0.14 ± 0.09 | 0.43 ± 0.09 | 0.78 ± 0.03 | 0.13 ± 0.03 | 0.83 ± 0.12 | 0.43 ± 0.04 | 0.54 ± 0.06 | 0.71 ± 0.19 | 0.84 ± 0.07 | 0.30 ± 0.03 | 0.23 ± 0.03 | 0.50 ± 0.13 | 0.53 ± 0.05 | 0.58 | 1.04 ± 0.13 | 0.54 ± 0.02 | 0.40 ± 0.13 | 1.04 ± 0.11 | 0.99 |
| Crude protein (N-Factor 6.25) | 29.4 ± 0.4 | 15.10 ± 0.5 | 15.23 ± 0.04 | 21.80 ± 0.05 | 32.1 ± 0.2 | 19.06 ± 0.08 | 30.4 ± 0.8 | 20.0 ± 0.1 | 24.4 ± 0.3 | 34.7 ± 0.5 | 31.0 ± 0.4 | 25.3 ± 0.2 | 15.94 ± 0.07 | 23.6 ± 0.5 | 13.23 ± 0.09 | 0.41 | 42.0 ± 0.3 | 40.2 ± 0.1 | 42.4 ± 0.6 | 30.2 ± 0.1 | 0.29 |
| Crude protein (N-Factor 4.97) | 23.4 ± 0.3 | 12.0 ± 0.4 | 12.11 ± 0.03 | 17.34 ± 0.04 | 25.5 ± 0.2 | 15.15 ± 0.06 | 30.4 ± 0.8 | 15.89 ± 0.08 | 19.4 ± 0.3 | 27.6 ± 0.4 | 24.6 ± 0.3 | 20.1 ± 0.2 | 12.67 ± 0.06 | 18.7 ± 0.4 | 10.52 ± 0.07 | 0.41 | 33.4 ± 0.3 | 32.00 ± 0.08 | 33.7 ± 0.5 | 24.0 ± 0.1 | 0.29 |
| Crude protein (Specific N-Factor) | 22.7 ± 0.3 | 13.2 ± 0.4 | 10.67 ± 0.03 | 16.29 ± 0.04 | 24.0 ± 0.2 | 16.7 ± 0.1 | 25.0 ± 0.6 | 14.6 ± 0.1 | 17.3 ± 0.2 | 26.4 ± 0.4 | 24.2 ± 0.3 | 22.7 ± 0.2 | 12.3 ± 0.1 | 17.5 ± 0.3 | 10.2 ± 0.1 | 0.29 | 32.0 ± 0.3 | 30.7 ± 0.1 | 20.5 ± 0.1 | 26.7 ± 0.4 | 0.04 |
| Pure protein (Specific N-Factor) | 18.7 ± 0.1 | 12.3 ± 0.2 | 10.0 ± 0.4 | 14.3 ± 0.4 | 20.31 ± 0.01 | 16.0 ± 0.2 | 20.68 ± 0.01 | 12.6 ± 0.2 | 14.9 ± 0.2 | 23.1 ± 0.8 | 20.1 ± 0.2 | 21.02 ± 0.04 | 11.2 ± 0.2 | 15.2 ± 0.5 | 7.7 ± 0.2 | 0.45 | 27.1 ± 0.5 | 28.16 ± 0.01 | 16.0 ± 0.5 | 25.1 ± 0.3 | 0.04 |
| Total fiber (Specific N-Factor) | 41.2 ± 1.1 | 34.2 ± 0.2 | 21.0 ± 1.2 | 14.3 ± 0.1 | 20.6 ± 0.7 | 29.4 ± 0.4 | 29.4 ± 0.4 | 34.0 ± 1.6 | 25.1 ± 2.1 | 28.9 ± 0.7 | 12.7 ± 0.5 | 40.5 ± 2.2 | 28.0 ± 0.3 | 34.6 ± 2.4 | 27.5 ± 1.3 | 0.68 | 32.6 ± 1.0 | 23.8 ± 0.8 | 36.6 ± 0.7 | 40.4 ± 0.5 | 0.27 |
2.3. Total Fat and Fatty Acids
| Species | Chrysotila carterae | Eustigmatos sp. | Microchloropsis salina | Nannochloropsis limnetica | Nitzschia palea | Phaeodactylum tricornutum | Autumnella lusatica | Botryococcus braunii | Chlorococcum novae-angliae | Klebsormidium sp. | Myrmecia bisecta | Spongiochloris minor | Stichococcus sp. | Tetradesmus obliquus | Tetraselmis suecica | Chlorococcum novae-angliae | Microchloropsis salina | Spongiochloris minor | Tetradesmus obliquus | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kingdom | Cr | Pl | Pl | Cr | Pl | Pl | |||||||||||||||
| CP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | ◊ | GP | GP | GP | GP | O |
| Total fat | 12.9 ± 0.1 | 38.3 ± 0.2 | 53.0 ± 0.3 | 40.1 ± 0.3 | 8.64 ± 0.09 | 27.43 ± 0.04 | 20.6 ± 0.6 | 51.09 ± 0.54 | 5.85 ± 0.18 | 4.91 ± 0.11 | 6.22 ± 0.27 | 8.93 ± 0.06 | 33.87 ± 0.08 | 8.07 ± 0.19 | 9.21 ± 0.14 | 0.03 | 6.78 ± 0.23 | 15.3 ± 0.3 | 7.71 ± 0.10 | 7.66 ± 0.22 | 0.17 |
| SFA | |||||||||||||||||||||
| C14:0 | 0.031 | 1.21 | 3.23 | 1.90 | 0.45 | 2.16 | 0.037 | 0.44 | 0.17 | 0.054 | 0.010 | 0.069 | 0.33 | 0.022 | 0.028 | 0.01 | 0.15 | 1.10 | 0.015 | 0.019 | 0.37 |
| C16:0 | 3.34 | 10.9 | 22.8 | 13.4 | 1.65 | 7.72 | 3.91 | 11.69 | 1.22 | 1.88 | 1.11 | 1.59 | 8.01 | 1.50 | 2.26 | 0.06 | 1.57 | 3.48 | 1.09 | 1.41 | 0.27 |
| C18:0 | 0.16 | 0.52 | 0.56 | 0.65 | 0.065 | 0.19 | 0.35 | 0.64 | 0.26 | 0.14 | 0.044 | 0.26 | 0.81 | 0.062 | 0.047 | 0.64 | 0.19 | 0.15 | 0.14 | 0.076 | 0.14 |
| C20:0 | <LOQ | 0.022 | <LOQ | 0.071 | 0.005 | 0.019 | 0.065 | 0.93 | 0.029 | 0.021 | 0.006 | 0.052 | 0.14 | <LOQ | <LOQ | 0.29 | 0.017 | 0.013 | 0.014 | 0.004 | 0.47 |
| C22:0 | <LOQ | <LOQ | <LOQ | <LOQ | 0.009 | 0.021 | 0.10 | 0.52 | 0.012 | 0.15 | 0.006 | 0.016 | 0.075 | 0.020 | <LOQ | 0.03 | 0.006 | 0.001 | 0.006 | 0.018 | 0.14 |
| C24:0 | 0.011 | <LOQ | <LOQ | <LOQ | 0.070 | 0.11 | 0.33 | <LOQ | 0.016 | 0.037 | 0.061 | 0.049 | 0.20 | 0.012 | 0.007 | 0.26 | 0.007 | 0.001 | 0.023 | 0.013 | 0.47 |
| MUFA | |||||||||||||||||||||
| C16:1n7 | 0.36 | 18.8 | 13.3 | 10.4 | 2.85 | 9.15 | 0.063 | 2.71 | 0.078 | 0.068 | 0.047 | 0.29 | 0.034 | 0.058 | 0.037 | 0.002 | 0.067 | 3.31 | 0.033 | 0.060 | 0.38 |
| C17:1n7 | <LOQ | <LOQ | 0.15 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | 0.003 | 0.032 | 0.014 | 0.016 | <LOQ | 0.033 | <LOQ | X | 0.008 | 0.078 | 0.014 | 0.030 | 0.47 |
| C18:1n9 | 2.82 | 3.34 | 6.98 | 9.08 | 0.053 | 1.84 | 5.29 | 15.8 | 0.14 | 0.16 | 0.99 | 3.38 | 13.6 | 1.55 | 2.46 | 0.91 | 0.19 | 0.63 | 0.65 | 1.39 | 0.14 |
| C18:1n7 | 0.39 | 0.24 | <LOQ | 0.17 | 0.41 | 0.13 | 0.17 | 0.98 | 0.97 | <LOQ | 0.40 | 0.063 | 0.083 | 0.066 | 0.38 | 0.95 | 0.85 | 0.058 | 0.070 | 0.063 | 0.70 |
| C20:1n9 | <LOQ | <LOQ | 0.002 | 0.030 | <LOQ | <LOQ | 0.065 | 0.58 | 0.014 | <LOQ | 0.017 | 0.050 | 0.26 | 0.015 | 0.12 | 0.02 | 0.015 | 0.005 | 0.029 | 0.014 | 0.72 |
| n6-PUFA | |||||||||||||||||||||
| C18:2n6 | 0.88 | 0.83 | 0.33 | 0.95 | 0.026 | 0.29 | 2.93 | 1.69 | 0.66 | 0.72 | 0.83 | 1.33 | 4.44 | 0.78 | 0.94 | 0.10 | 1.22 | 0.12 | 0.86 | 0.67 | 0.72 |
| C18:3n6 | 0.049 | 0.042 | 0.11 | 0.064 | 0.040 | 0.20 | 0.170 | <LOQ | 0.44 | 0.028 | 0.018 | 0.034 | 0.080 | 0.078 | 0.069 | 0.64 | 0.48 | 0.031 | 0.023 | 0.067 | 0.47 |
| C20:2n6 | 0.039 | <LOQ | <LOQ | 0.028 | <LOQ | 0.026 | <LOQ | <LOQ | 0.005 | 0.010 | 0.021 | 0.001 | 0.15 | <LOQ | 0.012 | 0.86 | 0.004 | 0.004 | 0.007 | 0.001 | 0.47 |
| C20:3n6 | 0.014 | 0.089 | <LOQ | 0.10 | 0.009 | 0.032 | <LOQ | <LOQ | <LOQ | 0.026 | 0.020 | 0.002 | 0.25 | <LOQ | 0.017 | 0.28 | <LOQ | 0.032 | <LOQ | <LOQ | X |
| C20:4n6 | 0.043 | 0.15 | 1.01 | 0.77 | 0.89 | 0.70 | <LOQ | <LOQ | <LOQ | 0.030 | 0.68 | 0.019 | 0.50 | 0.001 | 0.21 | 0.01 | <LOQ | 0.47 | <LOQ | 0.001 | 0.37 |
| n3-PUFA | |||||||||||||||||||||
| C18:3n3 | 1.23 | 0.21 | 0.009 | <LOQ | 0.068 | 0.023 | 3.25 | 6.70 | 0.51 | 0.076 | 0.44 | 0.74 | 2.87 | 1.34 | 0.46 | 0.01 | 0.71 | <LOQ | 2.59 | 1.10 | 0.72 |
| C20:5n3 | 0.25 | 1.24 | 1.70 | 1.68 | 0.51 | 2.78 | <LOQ | 1.08 | <LOQ | 0.015 | 0.091 | 0.04 | 0.10 | 0.004 | 0.50 | 0.005 | 0.001 | 2.97 | <LOQ | 0.008 | 0.41 |
| C22:6n3 | 0.86 | <LOQ | <LOQ | <LOQ | 0.044 | 0.15 | 0.014 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | X | <LOQ | <LOQ | <LOQ | <LOQ | X |
| Sum | |||||||||||||||||||||
| SFA | 3.54 | 12.6 | 26.8 | 16.1 | 2.26 | 10.2 | 4.79 | 14.3 | 1.70 | 2.28 | 1.23 | 2.04 | 9.56 | 1.62 | 2.34 | 0.045 | 1.93 | 4.86 | 1.29 | 1.54 | 0.27 |
| MUFA | 3.56 | 22.3 | 20.5 | 19.8 | 3.31 | 11.1 | 5.59 | 20.1 | 1.21 | 0.25 | 1.46 | 3.80 | 14.0 | 1.72 | 3.00 | 0.06 | 1.13 | 4.10 | 0.79 | 1.55 | 0.07 |
| PUFA | 3.51 | 2.57 | 3.15 | 3.59 | 1.59 | 4.19 | 6.37 | 9.47 | 1.62 | 0.93 | 2.10 | 2.17 | 8.40 | 2.20 | 2.21 | 0.64 | 2.41 | 3.64 | 3.48 | 1.85 | 0.14 |
| n6-PUFA | 1.16 | 1.12 | 1.45 | 1.92 | 0.97 | 1.24 | 3.10 | 1.69 | 1.11 | 0.84 | 1.56 | 1.39 | 5.43 | 0.85 | 1.25 | 0.48 | 1.70 | 0.66 | 0.89 | 0.74 | 0.47 |
| n3-PUFA | 2.35 | 1.46 | 1.71 | 1.68 | 0.62 | 2.95 | 3.27 | 7.78 | 0.51 | 0.09 | 0.54 | 0.78 | 2.97 | 1.34 | 0.96 | 0.64 | 0.71 | 2.97 | 2.59 | 1.11 | 0.27 |
| n6/n3 | 0.50 | 0.77 | 0.85 | 1.14 | 1.55 | 0.42 | 0.95 | 0.22 | 2.18 | 9.15 | 2.92 | 1.77 | 1.83 | 0.64 | 1.30 | 0.48 | 2.41 | 0.22 | 0.34 | 0.66 | 0.47 |
| Others | 2.28 | 0.77 | 2.51 | 0.67 | 1.48 | 1.87 | 3.81 | 7.22 | 1.32 | 1.44 | 1.43 | 0.91 | 1.91 | 2.53 | 1.66 | 0.10 | 1.31 | 2.72 | 2.15 | 2.72 | 0.14 |
2.4. Total Carotenoids and Total Chlorophyll
2.5. Main Elements
2.6. Minerals, Trace Elements and Heavy Metals
| Species | Chrysotila carterae | Eustigmatos sp. | Microchloropsis salina | Nannochloropsis limnetica | Nitzschia palea | Phaeodactylum tricornutum | Autumnella lusatica | Botryococcus braunii | Chlorococcum novae-angliae | Klebsormidium sp. | Myrmecia bisecta | Spongiochloris minor | Stichococcus sp. | Tetradesmus obliquus | Tetraselmis suecica | Chlorococcum novae-angliae | Microchloropsis salina | Spongiochloris minor | Tetradesmus obliquus | RDI and TDI | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kingdom | Cr | Pl | Pl | Cr | Pl | Pl | ||||||||||||||||
| CP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | SP | ◊ | GP | GP | GP | GP | O | |
| Main elements | ||||||||||||||||||||||
| C (g/100 g) | 43.47 ± 0.30 | 54.45 ± 0.20 | 58.813 ± 0.005 | 53.97 ± 0.39 | 25.13 ± 0.10 | 48.38 ± 0.14 | 49.51 ± 0.51 | 67.99 ± 0.93 | 43.77 ± 0.74 | 42.91 ± 0.33 | 34.69 ± 0.41 | 43.59 ± 0.91 | 50.09 ± 0.47 | 44.81 ± 0.79 | 40.02 ± 0.14 | 0.75 | 43.67 ± 0.70 | 48.73 ± 0.26 | 42.49 ± 0.80 | 46.42 ± 1.02 | 0.94 | X |
| H2 (g/100 g) | 6.23 ± 0.05 | 8.18 ± 0.03 | 9.01 ± 0.01 | 8.08 ± 0.02 | 4.06 ± 0.02 | 7.17 ± 0.02 | 7.05 ± 0.06 | 9.63 ± 0.18 | 6.60 ± 0.08 | 6.18 ± 0.04 | 5.18 ± 0.05 | 6.62 ± 0.17 | 7.45 ± 0.08 | 6.73 ± 0.10 | 6.21 ± 0.03 | 0.52 | 6.42 ± 0.11 | 7.23 ± 0.05 | 6.55 ± 0.14 | 6.74 ± 0.16 | 0.88 | X |
| S (g/100 g) | 0.96 ± 0.02 | 0.22 ± 0.04 | 0.32 ± 0.03 | 0.37 ± 0.12 | 0.52 ± 0.06 | 0.73 ± 0.04 | 0.55 ± 0.04 | 0.21 ± 0.04 | 0.43 ± 0.03 | 0.39 ±0.03 | 0.39 ± 0.04 | 0.38 ± 0.04 | 0.35 ±0.04 | 0.42 ±0.06 | 1.13 ±0.02 | 0.65 | 0.60 ±0.01 | 0.58 ±0.04 | 0.34 ±0.01 | 0.48 ±0.03 | 0.93 | X |
| Minerals | ||||||||||||||||||||||
| Mg (mg/100 g) | 496 ± 5 | 276 ± 2 | 219 ± 1 | 146.7 ± 0.5 | 331 ± 6 | 333 ± 3 | 334 ± 4 | 74.0 ± 1.6 | 218 ± 2 | 183 ± 1 | 1065 ± 28 | 188.5 ± 0.3 | 331 ± 3 | 226 ± 1 | 559 ± 3 | 0.15 | 319 ± 4 | 333.1 ± 0.8 | 169 ± 2 | 295 ± 18 | 0.005 | 300–350 mg |
| Ca (mg/100 g) | 258 ± 5 | 240.1 ± 0.5 | 80.4 ± 1.4 | 109 ± 4 | 21.8 ± 2.5 | 268 ± 1 | 33.9 ±1.6 | 15.4 ± 0.9 | 188 ± 1 | 81.5 ± 0.2 | 166 ± 8 | 149 ± 2 | 70 ± 1 | 233 ± 3 | 1707 ± 19 | 0.04 | 182.4 ± 0.6 | 197 ± 3 | 115.0 ± 0.9 | 151 ± 8 | 0.86 | 1000 mg |
| Trace elements | ||||||||||||||||||||||
| Mn (mg/100 g) | 5.82 ± 0.11 | 1.615 ± 0.008 | 1.582 ± 0.004 | 17.49 ± 0.03 | 6.23 ± 0.08 | 5.04 ± 0.02 | 3.26 ±0.01 | 1.96 ± 0.01 | 1.00 ± 0.03 | 1.886 ± 0.007 | 23.0 ± 1.5 | 0.76 ± 0.01 | 3.79 ± 0.03 | 0.7 ± 0.01 | 2.49 ± 0.01 | 0.82 | 1.53 ± 0.03 | 3.76 ± 0.02 | 0.90 ± 0.03 | 1.12 ± 0.06 | 0.01 | 2–5 mg |
| Fe (mg/100 g) | 411 ± 5 | 247.1 ± 0.4 | 70.6 ± 0.5 | 328 ± 4 | 441 ± 18 | 217 ± 1 | 205.4 ± 0.9 | 90.3 ± 1.3 | 135 ± 2 | 203.9 ± 0.8 | 1359 ± 57 | 122 ± 2.3 | 214 ± 1 | 92 ± 3 | 117.8 ± 0.3 | 0.33 | 205 ± 6 | 110.7 ± 0.4 | 124 ± 2 | 98.6 ± 4.2 | 0.01 | 10–15 mg |
| Cu (mg/100 g) | 1.05 ± 0.03 | 0.37 ± 0.01 | 0.290 ± 0.002 | 1.13 ± 0.04 | 3.90 ± 0.05 | 4.42 ± 0.01 | 0.447 ±0.002 | 0.31 ± 0.01 | 0.33 ± 0.01 | 0.633 ± 0.005 | 7.74 ± 0.12 | 0.48 ± 0.01 | 1.02 ± 0.01 | 0.380 ± 0.001 | 0.57 ± 0.01 | 0.70 | 0.150 ± 0.002 | 0.519 ± 0.001 | 0.383 ± 0.006 | 0.56 ± 0.02 | 0.21 | 1.0–1.5 mg |
| Zn (mg/100 g) | 5.48 ± 0.09 | 1.68 ± 0.01 | 1.61 ± 0.03 | 1.51 ± 0.01 | 3.87 ± 0.06 | 1.60 ± 0.02 | 1.85 ±0.02 | 1.37 ± 0.02 | 0.77 ± 0.02 | 0.54 ± 0.02 | 2.61 ± 0.03 | 0.62 ± 0.06 | 1.22 ± 0.02 | 0.501 ± 0.001 | 1.69 ± 0.04 | 0.78 | 0.71 ± 0.03 | 3.18 ± 0.02 | 0.70 ± 0.01 | 1.26 ± 0.01 | 0.02 | 7–16 mg |
| Se (μg/100 g) | 19.6 ± 2.9 | 10.2 ± 0.8 | 7.80 ± 1.03 | 132 ± 10 | 4.43 ± 0.91 | 3.45 ± 0.76 | 0.26 ±0.08 | 17.6 ± 1.3 | 7.89 ± 2.08 | 36.5 ± 1.4 | 9.19 ± 0.81 | 1.87 ± 0.23 | 20.6 ± 0.4 | 3.40 ± 1.10 | 46.1 ± 1.9 | 0.08 | 5.30 ± 0.12 | 9.86 ± 0.71 | 0.66 ± 0.03 | 2.33 ± 0.49 | 0.59 | 60–70 µg |
| Ni (mg/100 g) | 12.5 ± 0.3 | 2.66 ± 0.01 | 1.11 ± 0.01 | 7.58 ± 0.01 | 7.63 ± 0.11 | 6.47 ± 0.05 | 3.65 ±0.03 | 2.25 ± 0.03 | 1.31 ± 0.04 | 5.45 ± 0.03 | 141 ± 5 | 0.64 ± 0.03 | 3.49 ± 0.01 | 0.494 ± 0.001 | 3.13 ± 0.02 | 0.20 | 0.41 ± 0.01 | 0.48 ± 0.04 | 0.140 ± 0.002 | 0.311 ± 0.006 | 0.01 | 0.025–0.035 mg |
| Mo (mg/100 g) | 3.17 ± 0.42 | 1.363 ± 0.008 | 0.36 ± 0.01 | 3.98 ± 0.06 | 4.83 ± 0.25 | 2.02 ± 0.02 | 2.16 ±0.02 | 0.94 ± 0.06 | 0.74 ± 0.01 | 1.946 ± 0.009 | 5.20 ± 0.27 | 0.39 ± 0.02 | 3.08 ± 0.07 | 0.379 ± 0.001 | 1.35 ± 0.01 | 0.94 | 0.31 ± 0.01 | 0.33 ± 0.01 | 0.184 ± 0.001 | 0.275 ± 0.009 | 0.01 | 0.05–0.10 mg |
| I2 (μg/100 g) | 840 ±23 | 18.9 ± 5.0 | 72.4 ± 5.1 | 11.6 ± 1.5 | 21.6 ± 3.8 | 65.4 ± 8.2 | 39.3 ± 2.9 | 7.70 ± 0.4 | 41.2 ± 29.4 | 26.9 ± 5.2 | 20.4 ± 1.0 | 47.6 ± 23.8 | 70.0 ± 7.1 | 38.6 ± 16.3 | 62.1 ± 1.5 | 0.99 | 33.0 ± 27.3 | 71.6 ± 1.6 | 38.9 ± 19.0 | 33.3 ± 20.6 | 0.02 | 180–200 µg |
| Heavy metals **** | ||||||||||||||||||||||
| As (μg/100 g) | 25.5 ± 1.9 | 8.57 ± 0.36 | 129 ± 2 | 11.5 ± 0.3 | 17.9 ± 0.8 | 59.1 ± 0.8 | 10.1 ± 0.2 | 4.74 ± 0.35 | 6.47 ±0.13 | 9.54 ± 0.17 | 37.4 ± 0.8 | 2.93 ± 0.13 | 9.06 ± 0.44 | 2.65 ± 0.03 | 14.7 ± 0.3 | 0.01 | 14.2 ± 1.1 | 234 ± 3 | 2.52 ± 0.02 | 2.12 ± 0.07 | 0.07 | X |
| Cd (mg/100 g) | 0.175 ± 0.003 | 0.191 ± 0.001 | 0.237 ± 0.003 | 2.23 ± 0.02 | 0.078 ± 0.001 | 0.016 ± 0.001 | 0.337 ±0.003 | 1.48 ± 0.01 | 0.38 ± 0.03 | 1.551 ± 0.008 | 0.563 ± 0.001 | 0.158 ± 0.06 | 0.117 ± 0.002 | 0.131 ± 0.003 | 1.25 ± 0.01 | 0.65 | 0.375 ± 0.004 | 0.197 ± 0.001 | 0.165 ± 0.009 | 0.113 ± 0.001 | 0.05 | <0.1 mg/ 100 g |
| Hg (μg/100 g) | 0.80 ± 0.12 | 0.40 ± 0.08 | 0.59 ± 0.07 | 0.62 ± 0.01 | 2.04 ± 0.22 | 0.504 ± 0.007 | 0.319 ±0.003 | 0.45 ± 0.10 | 1.53 ± 0.01 | 0.62 ± 0.04 | 1.58 ± 0.26 | 1.02 ± 0.05 | 0.34 ± 0.06 | 0.97 ± 0.13 | 0.38 ± 0.13 | 0.53 | 1.41 ± 0.23 | 1.16 ± 0.08 | 0.63 ± 0.02 | 0.56 ± 0.04 | 0.95 | <10 μg/ 100 g |
| Pb (mg/100 g) | 0.54 ± 0.01 | 0.700 ± 0.001 | 0.110 ± 0.005 | 15.23 ± 0.07 | 0.338 ± 0.007 | 0.122 ± 0.003 | 2.51 ±0.02 | 0.790 ± 0.002 | 3.41 ± 0.23 | 0.936 ± 0.007 | 2.20 ± 0.08 | 1.51 ± 0.17 | 1.56 ± 0.01 | 1.18 ± 0.18 | 11.26 ± 0.06 | 0.006 | 2.33 ± 0.18 | 0.167 ± 0.002 | 0.69 ± 0.10 | 0.169 ± 0.021 | 0.05 | <0.3 mg/ 100 g |
3. Discussion
3.1. Variability between Kingdoms and Genera
3.2. Protein and Amino Acids
3.3. Dietary Fibers
3.4. Lipids
3.5. Pigments
3.6. Minerals and Trace Elements
3.7. Heavy Metals
3.8. Multi-Criteria Analysis of the Biomass Suitability for Nutrition
4. Materials and Methods
4.1. Microalgae Biomass
4.2. Amino Acid and Ammonium Quantification
4.3. N-Factor Calculation
4.4. Macronutrients
4.5. Fatty Acid Analysis
4.6. Total Carotenoids and Chlorophylls
4.7. Element Analysis (CHS)
4.8. Minerals, Trace Elements and Heavy Metals
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sandgruber, F.; Gielsdorf, A.; Baur, A.C.; Schenz, B.; Muller, S.M.; Schwerdtle, T.; Stangl, G.I.; Griehl, C.; Lorkowski, S.; Dawczynski, C. Variability in macro- and micronutrients of 15 commercially available microalgae powders. Mar. Drugs 2021, 19, 310. [Google Scholar] [PubMed]
- European Commission. Commission Implementing Regulation (EU) 2017/2470 of 20 December 2017 Establishing the Union List of Novel Foods in Accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on Novel Foods; Official Journal of the European Union: Brussels, Belgium, 2017. [Google Scholar]
- Pierre, G.; Delattre, C.; Dubessay, P.; Jubeau, S.; Vialleix, C.; Cadoret, J.-P.; Probert, I.; Michaud, P. What is in store for eps microalgae in the next decade? Molecules 2019, 24, 4296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richmond, A. Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Wiley Online Library: Hoboken, NJ, USA, 2004; Volume 577. [Google Scholar]
- You, J.; Mallery, K.; Mashek, D.G.; Sanders, M.; Hong, J.; Hondzo, M. Microalgal swimming signatures and neutral lipids production across growth phases. Biotechnol. Bioeng. 2020, 117, 970–980. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Muys, M.; Vermeir, P.; D’Adamo, S.; Vlaeminck, S.E. Light regime and growth phase affect the microalgal production of protein quantity and quality with dunaliella salina. Bioresour. Technol. 2019, 275, 145–152. [Google Scholar] [CrossRef]
- Lourenço, S.O.; Barbarino, E.; Marquez, U.M.L.; Aidar, E. Distribution of intracellular nitrogen in marine microalgae: Basis for the calculation of specific nitrogen-to-protein conversion factors. J. Phycol. 1998, 34, 798–811. [Google Scholar] [CrossRef]
- Choudhary, A.; Karmakar, R.; Kundu, K.; Dahake, V. “Algal” biodiesel: Future prospects and problems. Water Eenrgy Int. 2011, 68, 44–51. [Google Scholar]
- Hodgson, P.A.; Henderson, R.J.; Sargent, J.R.; Leftley, J.W. Patterns of variation in the lipid class and fatty acid composition of nannochloropsis oculata (eustigmatophyceae) during batch culture. J. Appl. Phycol. 1991, 3, 169–181. [Google Scholar]
- Schwarzhans, J.-P.; Cholewa, D.; Grimm, P.; Beshay, U.; Risse, J.-M.; Friehs, K.; Flaschel, E. Dependency of the fatty acid composition of euglena gracilis on growth phase and culture conditions. J. Appl. Phycol. 2015, 27, 1389–1399. [Google Scholar]
- Tossavainen, M.; Ilyass, U.; Ollilainen, V.; Valkonen, K.; Ojala, A.; Romantschuk, M. Influence of long term nitrogen limitation on lipid, protein and pigment production of euglena gracilis in photoheterotrophic cultures. PeerJ 2019, 7, e6624. [Google Scholar]
- Boussiba, S.; Bing, W.; Yuan, J.-P.; Zarka, A.; Chen, F. Changes in pigments profile in the green alga haeamtococcus pluvialis exposed to environmental stresses. Biotechnol. Lett. 1999, 21, 601–604. [Google Scholar]
- Niestroj, I. Praxis der Orthomolekularen Medizin: Physiologische Grundlagen. In Therapie mit Mikronährstoffen; Georg Thieme Verlag: Stuttgart, Germany, 2000. [Google Scholar]
- Deutsche Gesellschaft für Ernährung (DGE); Österreichische Gesellschaft für Ernährung (ÖGE); Schweizerische Gesellschaft für Ernährung (SGE). Referenzwerte für die Nährstoffzufuhr; Deutsche Gesellschaft für Ernährung (DGE): Bonn, Germany, 2021. [Google Scholar]
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs; Official Journal of the European Union: Brussels, Belgium, 2006. [Google Scholar]
- Cavalier-Smith, T. Kingdom chromista and its eight phyla: A new synthesis emphasising periplastid protein targeting, cytoskeletal and periplastid evolution, and ancient divergences. Protoplasma 2018, 255, 297–357. [Google Scholar] [PubMed] [Green Version]
- Cavalier-Smith, T. Principles of protein and lipid targeting in secondary symbiogenesis: Euglenoid, dinoflagellate, and sporozoan plastid origins and the eukaryote family tree 1, 2. J. Eukaryot. Microbiol. 1999, 46, 347–366. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.; Seckbach, J. The Algae World; Springer: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Mišurcová, L.; Buňka, F.; Vávra Ambrožová, J.; Machů, L.; Samek, D.; Kráčmar, S. Amino acid composition of algal products and its contribution to rdi. Food Chem. 2014, 151, 120–125. [Google Scholar] [PubMed]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J. Appl. Phycol. 2015, 27, 1109–1119. [Google Scholar]
- Cai, Y.; Zhai, L.; Fang, X.; Wu, K.; Liu, Y.; Cui, X.; Wang, Y.; Yu, Z.; Ruan, R.; Liu, T.; et al. Effects of c/n ratio on the growth and protein accumulation of heterotrophic chlorella in broken rice hydrolysate. Biotechnol. Biofuels Bioprod. 2022, 15, 102. [Google Scholar]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar]
- Nosworthy, M.G.; Franczyk, A.J.; Medina, G.; Neufeld, J.; Appah, P.; Utioh, A.; Frohlich, P.; House, J.D. Effect of processing on the in vitro and in vivo protein quality of yellow and green split peas (Pisum sativum). J. Agric. Food Chem. 2017, 65, 7790–7796. [Google Scholar] [CrossRef]
- Matissek, R.; Baltes, W. Lebensmittelchemie; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Fang, H.; Zhuang, Z.; Huang, L.; Zhao, W.; Niu, J.J.F.I.N. Dietary klebsormidium sp. Supplementation improves growth performance, antioxidant and anti-inflammatory status, metabolism, and mid-intestine morphology of litopenaeus vannamei. Front. Nutr. 2022, 9, 857351. [Google Scholar] [CrossRef]
- Brown, M.R. The amino-acid and sugar composition of 16 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 1991, 145, 79–99. [Google Scholar] [CrossRef]
- Miao, G.; Zhu, C.; Wang, J.; Tan, Z.; Wang, L.; Liu, J.; Kong, L.; Sun, Y. Efficient one-pot production of 1,2-propanediol and ethylene glycol from microalgae (Chlorococcum sp.) in water. Green Chem. 2015, 17, 2538–2544. [Google Scholar]
- Ramaraj, R.; Kawaree, R.; Unpaprom, Y. Direct transesterification of microalga botryococcus braunii biomass for biodiesel production. Emergent Life Sci. Res. 2016, 2, 1–7. [Google Scholar]
- Teuling, E.; Wierenga, P.A.; Schrama, J.W.; Gruppen, H. Comparison of protein extracts from various unicellular green sources. J. Agric. Food Chem. 2017, 65, 7989–8002. [Google Scholar] [CrossRef] [Green Version]
- Pollio, A.; Aliotta, G.; Pinto, G.; Paterno, M.; Bevilacqua, A. Ecophysiological characters and biochemical composition of stichococcus bacillaris naegeli strains from low ph environments. Algol. Stud./Arch. Hydrobiol. 1997, 84, 129–143. [Google Scholar] [CrossRef]
- Oliveira, C.Y.B.; Oliveira, C.D.; Prasad, R.; Ong, H.C.; Araujo, E.; Nisha, S.; Galvez, A. A multidisciplinary review of tetradesmus obliquus: A microalga suitable for large-scale biomass production and emerging environmental applications. Rev. Aquac. 2021, 13, 25. [Google Scholar] [CrossRef]
- Psachoulia, P.; Chatzidoukas, C. Illumination policies for Stichococcus sp. Cultures in an optimally operating lab-scale pbr toward the directed photosynthetic production of desired products. Sustainability 2021, 13, 2489. [Google Scholar] [CrossRef]
- Abid, A.L.; Bchir, F.; Hamdi, M. Feasibility of carbon dioxide sequestration by Spongiochloris sp. microalgae during petroleum wastewater treatment in airlift bioreactor. Bioresour. Technol. 2017, 234, 297–302. [Google Scholar] [CrossRef]
- Andreeva, A.P.; Budenkova, E.; Babich, O.; Sukhikh, S.; Ulrikh, E.; Ivanova, S.; Prosekov, A.; Dolganyuk, V. Production, purification, and study of the amino acid composition of microalgae proteins. Molecules 2021, 26, 2767. [Google Scholar] [CrossRef]
- Wild, K.J.; Steingass, H.; Rodehutscord, M. Variability in nutrient composition and in vitro crude protein digestibility of 16 microalgae products. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1306–1319. [Google Scholar] [CrossRef] [PubMed]
- Lourenco, S.O.; Barbarino, E.; Lavin, P.L.; Marque, U.M.L.; Aidar, E. Distribution of intracellular nitrogen in marine microalgae: Calculation of new nitrogen-to-protein conversion factors. Eur. J. Phycol. 2004, 39, 17–32. [Google Scholar] [CrossRef]
- González López, C.V.; García, M.D.C.C.; Fernández, F.G.A.; Bustos, C.S.; Chisti, Y.; Sevilla, J.M.F. Protein measurements of microalgal and cyanobacterial biomass. Bioresour. Technol. 2010, 101, 7587–7591. [Google Scholar] [CrossRef]
- Metsoviti, M.N.; Katsoulas, N.; Karapanagiotidis, I.T.; Papapolymerou, G. Effect of nitrogen concentration, two-stage and prolonged cultivation on growth rate, lipid and protein content of chlorella vulgaris. J. Chem. Technol. Biotechnol. 2019, 94, 1466–1473. [Google Scholar] [CrossRef]
- Da Costa, F.; Le Grand, F.; Quéré, C.; Bougaran, G.; Cadoret, J.P.; Robert, R.; Soudant, P. Effects of growth phase and nitrogen limitation on biochemical composition of two strains of tisochrysis lutea. Algal Res. 2017, 27, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Soliman, G.A. Dietary fiber, atherosclerosis, and cardiovascular disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.K.; Singhania, R.R.; Awasthi, M.K.; Varjani, S.; Bhatia, S.K.; Tsai, M.-L.; Hsieh, S.-L.; Chen, C.-W.; Dong, C.-D. Emerging prospects of macro- and microalgae as prebiotic. Microb. Cell Factories 2021, 20, 112. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Michael, M.; Rajput; Patil, R. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Molino, A.; Iovine, A.; Casella, P.; Mehariya, S.; Chianese, S.; Cerbone, A.; Rimauro, J.; Musmarra, D. Microalgae characterization for consolidated and new application in human food, animal feed and nutraceuticals. Int. J. Environ. Res. Public Health 2018, 15, 2436. [Google Scholar] [CrossRef] [Green Version]
- Germán-Báez, L.J.; Valdez-Flores, M.A.; Félix-Medina, J.V.; Norzagaray-Valenzuela, C.D.; Santos-Ballardo, D.U.; Reyes-Moreno, C.; Shelton, L.M.; Valdez-Ortiz, Á. Chemical composition and physicochemical properties of phaeodactylum tricornutum microalgal residual biomass. Food Sci. Technol. Int. 2017, 23, 681–689. [Google Scholar] [CrossRef]
- Zhuang, L.-L.; Azimi, Y.; Yu, D.; Wang, W.-L.; Wu, Y.-H.; Dao, G.-H.; Hu, H.-Y. Enhanced attached growth of microalgae scenedesmus. Lx1 through ambient bacterial pre-coating of cotton fiber carriers. Bioresour. Technol. 2016, 218, 643–649. [Google Scholar] [CrossRef]
- Kent, M.; Welladsen, H.M.; Mangott, A.; Li, Y. Nutritional evaluation of australian microalgae as potential human health supplements. PLoS ONE 2015, 10, e0118985. [Google Scholar] [CrossRef]
- Mutaf, T.; Oz, Y.; Kose, A.; Elibol, M.; Oncel, S.S. The effect of medium and light wavelength towards stichococcus bacillaris fatty acid production and composition. Bioresour. Technol. 2019, 289, 121732. [Google Scholar] [CrossRef]
- Schädler, T.; Caballero Cerbon, D.; de Oliveira, L.; Garbe, D.; Brück, T.; Weuster-Botz, D. Production of lipids with microchloropsis salina in open thin-layer cascade photobioreactors. Bioresour. Technol. 2019, 289, 121682. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Z.; Yu, C.; Yin, Y.; Zhou, G. Evaluation of the potential of 9 nannochloropsis strains for biodiesel production. Bioresour. Technol. 2014, 167, 503–509. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, H.; Zheng, L.; Cheng, W.; Gao, L.; Liu, T. Comparison of lipid and palmitoleic acid induction of tribonema minus under heterotrophic and phototrophic regimes by using high-density fermented seeds. Int. J. Mol. Sci. 2019, 20, 4356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Zhang, Y.; Zhou, W.; Noppol, L.; Liu, T. Mechanism and enhancement of lipid accumulation in filamentous oleaginous microalgae tribonema minus under heterotrophic condition. Biotechnol. Biofuels 2018, 11, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Mi, Y.; Zhao, C.; Wei, Q. A comprehensive review on carbon source effect of microalgae lipid accumulation for biofuel production. Sci. Total Environ. 2022, 806, 151387. [Google Scholar] [CrossRef]
- de Carvalho, C.C.C.R.; Caramujo, M.J. The various roles of fatty acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Bo, Y.; Liu, Y. Dietary total fat, fatty acids intake, and risk of cardiovascular disease: A dose-response meta-analysis of cohort studies. Lipids Health Dis. 2019, 18, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calder, P.C. Functional roles of fatty acids and their effects on human health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef]
- Viso, A.-C.; Marty, J.-C. Fatty acids from 28 marine microalgae. Phytochemistry 1993, 34, 1521–1533. [Google Scholar] [CrossRef]
- Krzemińska, I.; Nosalewicz, A.; Reszczyńska, E.; Pawlik-Skowrońska, B. Enhanced light-induced biosynthesis of fatty acids suitable for biodiesel production by the yellow-green alga eustigmatos magnus. Energies 2020, 13, 6098. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic acid: Physiological role, metabolism and nutritional implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef] [Green Version]
- Carta, G.; Murru, E.; Lisai, S.; Sirigu, A.; Piras, A.; Collu, M.; Batetta, B.; Gambelli, L.; Banni, S. Dietary triacylglycerols with palmitic acid in the sn-2 position modulate levels of n-acylethanolamides in rat tissues. PLoS ONE 2015, 10, e0120424. [Google Scholar] [CrossRef] [Green Version]
- Korbecki, J.; Bajdak-Rusinek, K. The effect of palmitic acid on inflammatory response in macrophages: An overview of molecular mechanisms. Inflamm. Res. 2019, 68, 915–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, E.A.; Farahat, L.A.; Abdel Aziz, Z.K.; Fatthallah, N.A.; Salah El Din, R.A. Evaluation of the potential for some isolated microalgae to produce biodiesel. Egypt. J. Pet. 2015, 24, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Dilia, P.; Kalsum, L.; Rusdianasari, R. Fatty acids from microalgae botryococcus braunii for raw material of biodiesel. J. Phys. Conf. Ser. 2018, 1095, 012010. [Google Scholar] [CrossRef]
- Shen, P.-L.; Wang, H.-T.; Pan, Y.-F.; Meng, Y.-Y.; Wu, P.-C.; Xue, S. Identification of characteristic fatty acids to quantify triacylglycerols in microalgae. Front. Plant Sci. 2016, 7, 162. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, T.; Fernandes, I.; Andrade, C.A.P.; Cordeiro, N. Changes in fatty acid biosynthesis in marine microalgae as a response to medium nutrient availability. Algal Res. 2016, 18, 314–320. [Google Scholar] [CrossRef]
- Lin, Q.; Gu, N.; Lin, J. Effect of ferric ion on nitrogen consumption, biomass and oil accumulation of a scenedesmus rubescens-like microalga. Bioresour. Technol. 2012, 112, 242–247. [Google Scholar] [CrossRef]
- Tan, Y.; Lin, J. Biomass production and fatty acid profile of a scenedesmus rubescens-like microalga. Bioresour. Technol. 2011, 102, 10131–10135. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Marine omega-3 (n-3) fatty acids for cardiovascular health: An update for 2020. Int. J. Mol. Sci. 2020, 21, 1362. [Google Scholar] [CrossRef] [Green Version]
- Grosso, G.; Galvano, F.; Marventano, S.; Malaguarnera, M.; Bucolo, C.; Drago, F.; Caraci, F. Omega-3 fatty acids and depression: Scientific evidence and biological mechanisms. Oxid. Med. Cell. Longev. 2014, 2014, 313570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Camarena-Gómez, M.T.; Piiparinen, J.; Spilling, K.; Yang, B. European union legislation on macroalgae products. Aquac. Int. 2021, 29, 487–509. [Google Scholar] [CrossRef]
- Rodriguez-Leyva, D.; Dupasquier, C.M.; McCullough, R.; Pierce, G.N. The cardiovascular effects of flaxseed and its omega-3 fatty acid, alpha-linolenic acid. Can. J. Cardiol. 2010, 26, 489–496. [Google Scholar] [CrossRef] [Green Version]
- Uauy, R.; Valenzuela, A. Marine oils as a source of omega-3 fatty acids in the diet: How to optimize the health benefits. Prog. Food Nutr. Sci. 1992, 16, 199–243. [Google Scholar]
- Mohammady, N.; Maghraby, D.; Ibrahim, E. Growth and oil production of nannochloropsis salina cultivated under multiple stressors. J. Pure Appl. Microbiol. 2014, 8, 2761–2772. [Google Scholar]
- Teh, K.Y.; Loh, S.H.; Aziz, A.; Takahashi, K.; Effendy, A.W.M.; Cha, T.S. Lipid accumulation patterns and role of different fatty acid types towards mitigating salinity fluctuations in chlorella vulgaris. Sci. Rep. 2021, 11, 438. [Google Scholar] [CrossRef] [PubMed]
- Fidalgo, J.P.; Cid, A.; Torres, E.; Sukenik, A.; Herrero, C. Effects of nitrogen source and growth phase on proximate biochemical composition, lipid classes and fatty acid profile of the marine microalga isochrysis galbana. Aquaculture 1998, 166, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Anne-Marie, K.; Yee, W.; Loh, S.H.; Aziz, A.; Cha, T.S. Effects of excess and limited phosphate on biomass, lipid and fatty acid contents and the expression of four fatty acid desaturase genes in the tropical selenastraceaen messastrum gracile se-mc4. Appl. Biochem. Biotechnol. 2020, 190, 1438–1456. [Google Scholar] [CrossRef]
- Wijendran, V.; Hayes, K.C. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu. Rev. Nutr. 2004, 24, 597–615. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Human requirement for n-3 polyunsaturated fatty acids. Poult. Sci. 2000, 79, 961–970. [Google Scholar] [CrossRef]
- da Silva, J.C.; Lombardi, A.T. Chlorophylls in microalgae: Occurrence, distribution, and biosynthesis. In Pigments from Microalgae Handbook; Jacob-Lopes, E., Queiroz, M.I., Zepka, L.Q., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–18. [Google Scholar]
- Marks, G.S. The biosynthesis of heme and chlorophyll. Bot. Rev. 1966, 32, 56–94. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Gatamaneni Loganathan, B.; Orsat, V.; Lefsrud, M.; Wu, B.S. A comprehensive study on the effect of light quality imparted by light-emitting diodes (leds) on the physiological and biochemical properties of the microalgal consortia of chlorella variabilis and scenedesmus obliquus cultivated in dairy wastewater. Bioprocess Biosyst. Eng. 2020, 43, 1445–1455. [Google Scholar] [CrossRef]
- Ambati, R.R.; Gogisetty, D.; Aswathanarayana, R.G.; Ravi, S.; Bikkina, P.N.; Bo, L.; Yuepeng, S. Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2018, 59, 1880–1902. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gálvez, A.; Viera, I.; Roca, M. Carotenoids and chlorophylls as antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, F.; Fanning, K.; Netzel, M.; Turner, W.; Li, Y.; Schenk, P.M. Profiling of carotenoids and antioxidant capacity of microalgae from subtropical coastal and brackish waters. Food Chem. 2014, 165, 300–306. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Sun, M.; Li, Q.; Li, A.; Zhang, C. Profiling of carotenoids in six microalgae (eustigmatophyceae) and assessment of their β-carotene productions in bubble column photobioreactor. Biotechnol. Lett. 2012, 34, 2049–2053. [Google Scholar] [CrossRef]
- Coesel, S.N.; Baumgartner, A.C.; Teles, L.M.; Ramos, A.A.; Henriques, N.M.; Cancela, L.; Varela, J.C.S. Nutrient limitation is the main regulatory factor for carotenoid accumulation and for psy and pds steady state transcript levels in dunaliella salina (chlorophyta) exposed to high light and salt stress. Mar. Biotechnol. 2008, 10, 602–611. [Google Scholar] [CrossRef]
- Di Lena, G.; Casini, I.; Lucarini, M.; Lombardi-Boccia, G. Carotenoid profiling of five microalgae species from large-scale production. Food Res. Int. 2019, 120, 810–818. [Google Scholar] [CrossRef]
- Pisal, D.S.; Lele, S. Carotenoid production from microalga, dunaliella salina. Indian J. Biotechnol. 2005, 4, 476–483. [Google Scholar]
- Ghaderpour, S.; Ahmadifard, N.; Agh, N.; Vahabzadeh, Z.; Estevez, A. Short-term enrichment of microalgae with inorganic selenium and zinc and their effects on the mineral composition of microalgae and marine rotifer brachionus plicatilis. Aquac. Nutr. 2021, 27, 2772–2785. [Google Scholar] [CrossRef]
- Masojídek, J.; Ranglová, K.; Lakatos, G.E.; Silva Benavides, A.M.; Torzillo, G. Variables governing photosynthesis and growth in microalgae mass cultures. Processes 2021, 9, 820. [Google Scholar] [CrossRef]
- Geider, R.J.; La Roche, J. The role of iron in phytoplankton photosynthesis, and the potential for iron-limitation of primary productivity in the sea. Photosynth. Res. 1994, 39, 275–301. [Google Scholar] [CrossRef] [PubMed]
- Black, J.R.; Yin, Q.-Z.; Casey, W.H. An experimental study of magnesium-isotope fractionation in chlorophyll-a photosynthesis. Geochim. Cosmochim. Acta 2006, 70, 4072–4079. [Google Scholar] [CrossRef] [Green Version]
- Vannucci, L.; Fossi, C.; Quattrini, S.; Guasti, L.; Pampaloni, B.; Gronchi, G.; Giusti, F.; Romagnoli, C.; Cianferotti, L.; Marcucci, G.; et al. Calcium intake in bone health: A focus on calcium-rich mineral waters. Nutrients 2018, 10, 1930. [Google Scholar] [CrossRef] [Green Version]
- Hodges, J.; Cao, S.; Cladis, D.; Weaver, C. Lactose intolerance and bone health: The challenge of ensuring adequate calcium intake. Nutrients 2019, 11, 718. [Google Scholar] [CrossRef] [Green Version]
- Elmadfa, P.D.I.; Muskat, P.D.E.; Fritzsche, D.; Meyer, A.L. Die Große gu Nährwert-Kalorien-Tabelle; GRÄFE UND UNZER Verlag GmbH: München, Germany, 2021. [Google Scholar]
- Volpe, S.L. Magnesium in disease prevention and overall health. Adv. Nutr. 2013, 4, 378s–383s. [Google Scholar] [CrossRef] [Green Version]
- Sebastiani, G.; Herranz Barbero, A.; Borrás-Novell, C.; Alsina Casanova, M.; Aldecoa-Bilbao, V.; Andreu-Fernández, V.; Pascual Tutusaus, M.; Ferrero Martínez, S.; Gómez Roig, M.D.; García-Algar, O. The effects of vegetarian and vegan diet during pregnancy on the health of mothers and offspring. Nutrients 2019, 11, 557. [Google Scholar] [CrossRef] [Green Version]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Kieliszek, M. Selenium–fascinating microelement, properties and sources in food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [Green Version]
- Lin, P.H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in wound healing modulation. Nutrients 2017, 10, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of zinc in immune system and anti-cancer defense mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, P.D.M.; Glomb, P.D.M.A. Moderne Lebensmittelchemie; Behr’s Verlag DE: Hamburg, Germany, 2015. [Google Scholar]
- Tripathy, A.; More, R.; Gupta, S.; Samuel, J.; Singh, J.; Prasad, R. Present and future prospect of algae: A potential candidate for sustainable pollution mitigation. Open Biotechnol. J. 2021, 15, 142–156. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar]
- Ratnaike, R.N. Acute and chronic arsenic toxicity. Postgrad. Med. J. 2003, 79, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Fabregas, J.; Herrero, C. Marine microalgae as a potential source of minerals in fish diets. Aquaculture 1986, 51, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, B.; Bhuyan, P.P.; Nayak, R.; Patra, S.; Behera, C.; Ki, J.-S.; Ragusa, A.; Lukatkin, A.S.; Jena, M. Microalgal phycoremediation: A glimpse into a sustainable environment. Toxics 2022, 10, 525. [Google Scholar] [CrossRef]
- Spain, O.; Plöhn, M.; Funk, C.J.P.P. The cell wall of green microalgae and its role in heavy metal removal. Physiol. Plant. 2021, 173, 526–535. [Google Scholar] [CrossRef]
- Matissek, R.; Steiner, G.; Fischer, M. Lebensmittelanalytik; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Lee, S.C.; Prosky, L.; Vries, J.W.D. Determination of total, soluble, and insoluble dietary fiber in foods—Enzymatic-gravimetric method, mes-tris buffer: Collaborative study. J. AOAC Int. 2020, 75, 395–416. [Google Scholar] [CrossRef]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Jaspars, E. Pigmentation of tobacco crown-gall tissues cultured in vitro in dependence of the composition of the medium. Physiol. Plant. 2006, 18, 933–940. [Google Scholar] [CrossRef]
- Rowan, K.S.; Press, C.U. Photosynthetic Pigments of Algae; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]

| Ranking | Name | Kingdom | CP | Positive Characteristics | Negative Characteristics |
|---|---|---|---|---|---|
| 1. | Chrysotila carterae | Cr | SP | ↑EAA, ↑↑↑Fiber, ↑↑Carotenoids, ↑↑↑DHA, ↓↓n6/n3, ↑↑Mg, ↑↑↑Ca, ↑Mn, ↑↑Fe, ↑↑↑Zn, ↑Se, ↑↑Ni, ↑↑Mo, ↑↑↑I2 | (↑↑As) |
| 2. | Microchloropsis salina | Cr | GP | ↑↑SEA, ↑↑↑EAA, ↑↑↑ Protein, ↑↑↑EPA, ↓↓↓n6/n3, ↑Mg, ↑Ca, ↑↑Zn, ↑I2 | (↑Hg), (↑↑↑As) |
| 3. | Phaeodactylum tricornutum | Cr | SP | ↑↑↑EPA, ↓↓n6/n3, ↑↑↑Carotenoids, ↑Mg, ↑↑Ca, ↑↑Cu, ↑I2 | ↑SFA, (↑↑As) |
| 4. | Myrmecia bisecta | Pl | SP | ↑↑Protein, ↑↑↑Mg, ↑↑↑Mn, ↑↑↑Fe, ↑↑↑Cu, ↑Zn, ↑↑↑Ni, ↑↑↑Mo | ↑↑n6/n3,(↑↑Pb, ↑↑Hg) (↑↑As) |
| 5. | Tetradesmus obliquus | Pl | GP | ↑↑↑SEA, ↑↑Fiber, ↑↑ALA, ↓↓n6/n3 | |
| 6. | Spongiochloris minor | Pl | GP | ↑↑Protein, ↑↑↑Fiber, ↓n6/n3 | |
| 7. | Eustigmatos sp. | Cr | SP | ↑↑Fiber, ↑EPA, ↓n6/n3, ↑↑Carotenoids | ↑SFA |
| 8. | Autumnella lusatica | Pl | SP | ↑SEA, ↑EAA, ↑↑Protein, ↑↑ALA, ↑↑↑Carotenoids, ↑Mg | (↑↑Pb) |
| 9. | Stichococcus sp. | Pl | SP | ↑↑EPA, ↑Mg, ↑Se, ↑Mo, ↑I2 | ↑n6/n3, |
| 10. | Nitzschia palea | Cr | SP | ↑↑Protein,↑Mg, ↑Mn, ↑↑Fe, ↑↑Cu, ↑↑Zn, ↑Ni, ↑↑Mo, | (↑↑↑Hg) |
| 11. | Botryococcus braunii | Pl | SP | ↑↑Fiber, ↑↑↑ALA, ↑EPA, ↑↑↑n6/n3 | ↑↑SFA, (↑↑Cd) |
| 12. | Tetradesmus obliquus | Pl | SP | ↑↑Fiber, ↓n6/n3, ↑↑Ca | (↑↑Hg) |
| 13. | Nannochloropsis limnetica | Cr | SP | ↑↑EPA, ↑↑↑Mn, ↑↑Fe, ↑↑↑Se, ↑Ni, ↑Mo | ↑↑SFA, (↑↑↑Cd, ↑↑↑Pb) |
| 14. | Spongiochloris minor | Pl | SP | ↑↑EAA, ↑↑Protein, ↑↑↑Fiber, ↑I2 | ↑n6/n3, (↑Hg) |
| 15. | Chlorococcum novae-angliae | Pl | GP | ↑↑SEA, ↑↑↑EAA, ↑↑↑Protein, ↑↑Fiber, ↑Mg | ↑↑n6/n3, (↑↑Hg), (↑↑ Pb) |
| 16. | Microchloropsis salina | Cr | SP | ↑↑EPA, ↓n6/n3, ↑I2 | ↑↑↑SFA, (↑↑↑As) |
| 17. | Tetraselmis suecica | Pl | SP | ↑↑Mg, ↑↑↑Ca, ↑Se, ↑↑I2 | (↑↑Cd, ↑↑↑Pb) |
| 18. | Klebsormidium sp. | Pl | SP | ↑↑EAA, ↑↑Protein, ↑Se | ↑↑↑n6/n3, (↑↑Cd) |
| 19. | Chlorococcum novae-angliae | Pl | SP | ↑I2 | ↑↑n6/n3,(↑↑Hg, ↑↑Pb) |
| Microalgae (Strain Number) | Kingdom | Phylum | Class | Habitat | Culture Medium | PBR | Point of Harvest |
|---|---|---|---|---|---|---|---|
| Chrysotila carterae (SAG 944-1) | Cr | Haptophyta | Coccolithophyceae | Marine | SWES | CF | SP |
| Eustigmatos sp. (KASC I-005) | Cr | Ochrophyta | Eustigmatophyceae | Aeroterrestrial | BBM | BC | SP |
| Microchloropsis salina (SAG 40.85) | Cr | Ochrophyta | Eustigmatophyceae | Marine | f/2 | BC | GP, SP |
| Nannochloropsis limnetica (SAG 18.99) | Cr | Ochrophyta | Eustigmatophyceae | Fresh water | OHM | BC | SP |
| Nitzschia palea (KASC I-007) | Cr | Bacillariophyta | Bacillariophyceae | Fresh water | BBM +Na2SiO3 | CF | SP |
| Phaeodactylum tricornutum (SAG 1090-1b) | Cr | Bacillariophyta | Bacillariophyta classis incertae sedis | Marine | 1/2 SWES | CF | SP |
| Autumnella lusatica (Hindak 2012/2) | Pl | Chlorophyta | Trebouxiophyceae | Fresh water | KUHL | BC | SP |
| Botryococcus braunii (University of Tokyo S. Okada) | Pl | Chlorophyta | Trebouxiophyceae | Fresh water | BG11 | BC | SP |
| Chlorococcum novae-angliae (SAG 5.85) | Pl | Chlorophyta | Chlorophyceae | Fresh water | ES | CF | GP, SP |
| Klebsormidium sp. (KASC I-008) | Pl | Charophyta | Klebsormidiophyceae | Aeroterrestrial | BBM/Šetlik (1:1) | BC | SP |
| Myrmecia bisecta (SAG 2043) | Pl | Chlorophyta | Trebouxiophyceae | Terrestrial | BBM | BC | SP |
| Spongiochloris minor (KASC 29.01) | Pl | Chlorophyta | Chlorophyceae | Terrestrial | BBM | CF | GP, SP |
| Stichococcus sp. (KASC I-30-01) | Pl | Chlorophyta | Trebouxiophyceae | Aeroterrestrial | BBM/Šetlik (2:1) | BC | SP |
| Tetradesmus obliquus (SAG 276-1) | Pl | Chlorophyta | Chlorophyceae | Fresh water | BBM | CF | GP, SP |
| Tetraselmis suecica (CCAP 66/38) | Pl | Chlorophyta | Chlorodendrophyceae | Marine | SWES | BC | SP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandgruber, F.; Gielsdorf, A.; Schenz, B.; Müller, S.M.; Schwerdtle, T.; Lorkowski, S.; Griehl, C.; Dawczynski, C. Variability in Macro- and Micronutrients of 15 Rarely Researched Microalgae. Mar. Drugs 2023, 21, 355. https://doi.org/10.3390/md21060355
Sandgruber F, Gielsdorf A, Schenz B, Müller SM, Schwerdtle T, Lorkowski S, Griehl C, Dawczynski C. Variability in Macro- and Micronutrients of 15 Rarely Researched Microalgae. Marine Drugs. 2023; 21(6):355. https://doi.org/10.3390/md21060355
Chicago/Turabian StyleSandgruber, Fabian, Annekathrin Gielsdorf, Benjamin Schenz, Sandra Marie Müller, Tanja Schwerdtle, Stefan Lorkowski, Carola Griehl, and Christine Dawczynski. 2023. "Variability in Macro- and Micronutrients of 15 Rarely Researched Microalgae" Marine Drugs 21, no. 6: 355. https://doi.org/10.3390/md21060355
APA StyleSandgruber, F., Gielsdorf, A., Schenz, B., Müller, S. M., Schwerdtle, T., Lorkowski, S., Griehl, C., & Dawczynski, C. (2023). Variability in Macro- and Micronutrients of 15 Rarely Researched Microalgae. Marine Drugs, 21(6), 355. https://doi.org/10.3390/md21060355