Qualitative and Quantitative Saponin Contents in Five Sea Cucumbers from the Indian Ocean
Abstract
:1. Introduction
2. Results
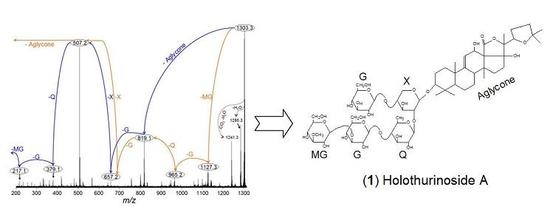
2.1. Qualitative study
2.2. Semi-quantitative study
3. Discussion
4. Experimental Section
4.1. Sampling
4.2. Extraction and purification of saponins
4.3. Mass spectrometry
4.4. Semi-quantitative study
Hemolytic activity
Orcinol reaction
Acknowledgements
References
- Nigrelli, RF. The effect of holothurin on fish, and mice with sarcoma 180. Zoologicia 1952, 37, 89–90. [Google Scholar]
- Yamanouchi, T. On the poisonous substance contained in holothurians. Publ Seto Mar Biol Lab 1955, 4, 183–203. [Google Scholar]
- Matsuno, T; Ishida, T. Distribution and seasonal variation of toxic principles of sea cucumber (Holothuria leucospilota Brandt). Experientia 1969, 25, 1261. [Google Scholar]
- Elyakov, GB; Stonik, VA; Levina, EV; Slanke, VP; Kuznetsova, TA; Levin, VS. Glycosides of marine invertebrates-I. A comparative study of the glycosides fraction of Pacific sea cucumbers. Comp Biochem Physiol 1973, 44, 325–336. [Google Scholar]
- Habermehl, G; Volkwein, G. Aglycones of the toxins from the Cuvierian organs of Holothuria forskali and a new nomenclature for the aglycones form Holothurioideae. Toxicon 1971, 9, 319–326. [Google Scholar]
- Stonik, VA; Kalinin, VI; Avilov, SA. Toxins from sea cucumbers (Holothuroids): chemical structures, properties, taxonomic distribution, biosynthesis and evolution. J Nat Toxins 1999, 8, 235–248. [Google Scholar]
- Stonik, VA; Elyakov, GB. Scheuer, PJ, Ed.; Secondary metabolites from echinoderms as chemotaxonomic markers. In Bioorganic Marine Chemistry; Springer-Verlag: Berlin, Germany, 1988; Volume 2, p. 43. [Google Scholar]
- Guo, M; Song, F; Liu, Z; Liu, S. Characterization of triterpenoidic saponin mixture in crude extracts from leaves of Acanthopanax senticosus harms by saponin structural correlation and mass spectrometry. Anal Chim Acta 2006, 557, 198–203. [Google Scholar]
- Garneau, FX; Harvey, O; Simard, JL; Apsimon, JW; Burnell, DJ; Himmelman, JH. The distribution of asterosaponins in various body components of the starfish Leptasterias polaris. Comp Biochem Physiol 1989, 92, 411–416. [Google Scholar]
- Kalinin, VI; Anisimov, MM; Prokofieva, NG; Avilov, SA; Afiyatullov, SH; Stonik, VA. Jangoux, M, Lawrence, LM, Eds.; Biological activities and biological role of triterpene glycosides from holothuroids (Echinodermata). In Echinoderm studies, 5th eEd; Balkema: Rotterdam, The Netherland, 1996; pp. 139–181. [Google Scholar]
- Kobayashi, M; Hori, M; Kan, K; Yasuzawa, T; Matsui, M; Suzuki, S; Kitagawa, I. Marine natural product. XXVII. Distribution of lanostane-type triterpene oligoglycosides in ten kinds of Okinawan sea cucumbers. Chem Pharm Bull 1991, 39, 2282–2287. [Google Scholar]
- Takemae, N; Nakaya, F; Motokawa, T. Low oxygen consumption and high body content of catch connective tissue contribution to low metabolic rate of sea cucumbers. Biol Bull 2009, 216, 45–54. [Google Scholar]
- Hamel, JF; Mercier, A. Cuvierian tubules in tropical holothurian: Usefulness and efficiency as a defence mechanism. Mar Freshwater Behav Physiol 2000, 33, 115–139. [Google Scholar]
- Flammang, P; Ribesse, J; Jangoux, M. Biomechanics of adhesion in sea cucumber Cuvierian tubules (Echinodermata, Holothuroidea). Integr Comp Biol 2002, 42, 1107–1115. [Google Scholar]
- Fang, S; Hao, C; Sun, W; Liu, Z; Liu, S. Rapid analysis of steroidal saponin mixture using electrospray ionization mass spectrometry combined with sequential tandem mass spectrometry. Rapid Commun Mass Spectrom 1998, 12, 589–594. [Google Scholar]
- Li, R; Zhou, Y; Wu, Z; Ding, L. ESI-QqTOF-MS/MS and APCI-IT-MS/MS analysis of steroid saponins from the rhizomes of Dioscorea panthaica. J Mass Spectrom 2006, 41, 1–22. [Google Scholar]
- Madl, T; Sterk, H; Mittelbach, M; Rechberger, GN. Tandem mass spectrometric analysis of a complex triterpene saponin mixture of Chenopodium quinoa. J Am Soc Mass Spectrom 2006, 17, 795–806. [Google Scholar]
- van Dyck, S; Gerbaux, P; Flammang, P. Elucidation of molecular diversity and body distribution of saponins in the sea cucumber Holothuria forskali (Echinodermata) by mass spectrometry. Comp Biochem Physiol 2009, 152, 124–134. [Google Scholar]
- Rodrigez, J; Castro, R; Riguera, R. Holothurinosides: new antitumour non sulphated triterpenoid glycosides from the sea cucumber Holothuria forskali. Tetrahedron 1991, 47, 4753–4762. [Google Scholar]
- Wu, J; Yi, Y-H; Zou, Z-R; Wu, H-M; Tang, H-F. Two new triterpene glycosides from sea cucumber Holothuria nobilis. Chin Trad Herb Drugs 2006, 37, 497–500. [Google Scholar]
- Ohta, T; Hikino, H. Structures of four new triterpenoidal oligosides, bivittoside A, B, C and D, from the sea cucumber Bohadschia bivittata Mitsukuri. Chem Pharm Bull 1981, 29, 282–285. [Google Scholar]
- Liu, B-S; Yi, Y-H; Li, L; Sun, P; Yuan, W-H; Sun, G-Q; Han, H; Xue, M. Arguside B and C, two new cytotoxic triterpene glycosides from the sea cucumber Bohadschia argus Jaeger. Chem Biodiv 2008, 5, 1288–1297. [Google Scholar]
- Sun, P; Liu, B-S; Yi, Y-H; Li, L; Gui, M; Tang, H-F; Zhang, D-Z; Zhang, S-L. A new cytotoxic lanostan-type triterpene glycoside from the sea cucumber Holothuria impatiens. Chem Biodiv 2007, 4, 450–457. [Google Scholar]
- Yuan, W-H; Yi, Y-H; Tang, H-F; Liu, B-S; Wang, Z-L; Sun, G-Q; Zhang, W; Li, L; Sun, P. Antifungal triterpene glycosides from the sea cucumber Bohadschia marmorata. Planta Med 2009, 75, 168–173. [Google Scholar]
- Kitagawa, I; Nishino, T; Kyogoku, Y. Structure of holothurine A. A pharmacologically active triterpene-oligoglycoside from the sea cucumber Holothuria leucospilota Brandt. Tetrahedron Lett 1979, 16, 1419–1422. [Google Scholar]
- Kitagawa, I; Kobayashi, M; Inamoto, T; Fuchida, M; Kyogoku, Y. Marine natural products. XIV. Structures of echinosides A and B, antifungal lanostane-oligosides from the sea cucumber Actinopyga echinites (Jaeger). Chem Pharm Bull 1985, 33, 5214–5224. [Google Scholar]
- Kitagawa, I; Nishino, T; Matsuno, T; Akutsu, H; Kyogoku, Y. Structure of holothurine B. A pharmacologically active triterpene-oligoglycoside from the sea cucumber Holothuria leucospilota Brandt. Tetrahedron Lett 1978, 11, 985–988. [Google Scholar]
- Silchenko, AS; Stonik, VA; Avilov, SA; Kalinin, VI; Kalinovsky, AI; Zaharenko, AM; Smirnov, AV; Mollo, E; Cimino, G. Holothurins B2, B3 and B4, new triterpene glycosides from Mediterranean sea cucumbers of the genus Holothuria. J Nat Prod 2005, 68, 564–567. [Google Scholar]
- Zhang, S-Y; Yi, Y-H; Tang, H-F. Bioactive triterpene glycosides from the sea cucumber Holothuria fuscocinerea. J Nat Prod 2006, 69, 1492–1495. [Google Scholar]
- Stonik, VA. Some triterpenoid and steroid derivatives from echinoderms and sponges. Pure Appl Chem 1986, 58, 423–436. [Google Scholar]
- Kitagawa, I; Kobayashi, M; Kyogoku, Y. Marine natural product. IX. Structural elucidation of triterpenoidal oligoglycosides from the Bahamean sea cucumber Actinopyga agassizi. Chem Pharm Bull 1981, 30, 2045–2050. [Google Scholar]
- Han, H; Yi, YH; Li, L; Wang, XH; Liu, BS; Sun, P; Pan, MX. A new triterpene glycoside from sea cucumber Holothuria leucospilota. Chin Chem Lett 2007, 18, 161–164. [Google Scholar]
- Han, H; Yi, YH; Liu, BL; Wang, XH; Pan, MX. Leucospilotaside C, a new sulphated triterpene glycoside from sea cucumber Holothuria leucospilota. Chin Chem Lett 2008, 19, 1462–1464. [Google Scholar]
- Burnell, DJ; Apsimon, JW. Echinoderm saponins. Mar Nat Prod Chem 1983, 5, 287–389. [Google Scholar]
- Hyman, L. The invertebrate: echinodermata The coelomate bilateria; McGraw-Hill Book Co: New York, NY, USA, 1955; Volume 4, p. 763. [Google Scholar]
- Mosher, C. Observation on evisceration and visceral regeneration in the sea-cucumber, Actinopyga agassizi Selenka. Zoologica 1956, 41, 17–26. [Google Scholar]
- VandenSpiegel, D; Jangoux, M. Fine structure and behaviour of the so-called Cuvierian organs on the holothuroid genus Actinopyga (Echinodermata). Acta Zool 1993, 74, 43–50. [Google Scholar]
- Lawrence, JM. Function of eponymous structures in echinoderms: a review. Can J Zool 2001, 79, 1251–1264. [Google Scholar]
- Samyn, Y; Appeltans, W; Kerr, AM. Phylogeny of Labidodemas and the Holothuriidae (Holothuroidea: Aspidochirotida) as inferred from morphology. Zool J Linnean Soc 2005, 144, 103–120. [Google Scholar]
- Féral, J-P; Cherbonnier, G. Guille, A, Laboute, P, Menou, J-L, Eds.; Les holothurides. In Guide des étoiles de mer, oursins et autres échinodermes du lagon de Nouvelle-Calédonie; Coll. Faune Tropicale: Orstom, Paris, France, 1986; p. 238. [Google Scholar]
- Kalinin, VI. System-theoretical (holistic) approach to the modelling of structural-functional relationships of biomolecules and their evolution: an example of triterpene glycosides from sea cucumbers (Echinodermata, Holothuroidea). J Theor Biol 2000, 206, 151–168. [Google Scholar]
- Campagnuolo, C; Fattorusso, E; Taglialatela-Scafati, O. Feroxosides A-B, two norlanostane tetraglycosides from the Caribbean sponge Ectyoplasia ferox. Tetrahedron 2001, 57, 4049–4055. [Google Scholar]
- Garneau, FX; Simard, JL; Harvey, O; Apsimon, JW; Girard, M. The structure of psoluthurin A, the major triterpene glycoside of the sea cucumber Psolus fabricii. Can J Chem 1983, 61, 1465–1471. [Google Scholar]
- Kalinin, VI; Prokofieva, NG; Likhatskaya, GN; Schentsova, EB; Agafonova, IG; Avilov, SA; Drozdova, OA. Hemolytic activities of triterpene glycosides from the holothurian order Dendrochirotida: some trends in the evolution of this group of toxins. Toxicon 1996, 34, 475–483. [Google Scholar]
- Mackie, AM; Lasker, R; Grant, PT. Avoidance reactions of a mollusc Buccinum undatum to saponin-like surface-active substances in extracts of the starfish Asterias rubens and Marthasterias glacialis. Comp Biochem Physiol 1968, 26, 415–428. [Google Scholar]
- Kabat, EA; Mayer, MM. Carbohydrate estimation. In Experimental Immunochemistry, 2nd ed; Thomas, C.C. Publisher: Springfield, IL, USA, 1967; p. 527. [Google Scholar]






| Saponin name | MW | S | Actinopyga echinites | Bohadschia subrubra | Holothuria atra | Holothuria leucospilota | Pearsonothuria graeffei | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-sulfated saponins | BW | CT | BW | CT | BW | BW | CT | BW | CT | |||
| Holothurinoside C | 1102 | 11 | x | [19] | ||||||||
| Desholothurin A (Nobiliside 2a)* | 1118 | 15 | x | x | x | [19,20] | ||||||
| Holothurinoside K1** | 1134 | 20 | x | |||||||||
| Holothurinoside E1 | 1264 | 16 | x | [18] | ||||||||
| Holothurinoside F | 1410 | 12 | x | [18] | ||||||||
| Bivittoside C | 1410 | 22 | x | [21] | ||||||||
| Impatienside A (Marmoratoside A)* | 1424 | 24 | X | x | [23,24] | |||||||
| Isomer | 1424 | x | ||||||||||
| Isomer | 1424 | x | ||||||||||
| Bivittoside D | 1426 | 23 | X | x | x | [21] | ||||||
| Isomer | 1426 | x | ||||||||||
| Holothurinoside H | 1440 | 13 | x | x | [18] | |||||||
| Holothurinoside H1 | 1440 | 17 | X | x | [18] | |||||||
| Isomer | 1440 | x | ||||||||||
| Isomer | 1440 | x | ||||||||||
| Arguside C | 1442 | 21 | x | [22] | ||||||||
| Holothurinoside I | 1456 | 14 | x | x | [18] | |||||||
| Holothurinoside I1 | 1456 | 18 | x | x | [18] | |||||||
| Isomer | 1456 | x | ||||||||||
| Isomer | 1456 | x | ||||||||||
| Isomer | 1456 | x | ||||||||||
| Holothurinoside J1** | 1472 | 19 | x | x | ||||||||
| Sulfated saponins | ||||||||||||
| Holothurin B3 | 866 | 6 | x | x | x | x | [28] | |||||
| Holothurin B1 | 868 | 4 | X | x | [26] | |||||||
| Holothurin B/B4*** | 882 | 3/7 | X | X | X | x | x | x | [27,28] | |||
| Holothurin B2 | 884 | 5 | x | x | [28] | |||||||
| Isomer | 884 | x | ||||||||||
| Fuscocineroside B/C*** | 1204 | 8/9 | x | X | x | x | [29] | |||||
| Isomer | 1204 | x | x | |||||||||
| Isomer | 1204 | x | ||||||||||
| Holothurin A2 | 1206 | 2 | x | X | x | [26] | ||||||
| Isomer | 1206 | x | ||||||||||
| Holothurin A | 1220 | 1 | x | X | X | X | X | [25] | ||||
| Species | Body compartment | Hemolytic activity (mg eq./g*) | Orcinol reaction (mg glycoside/g) |
|---|---|---|---|
| Actinopyga echinites | Body wall | 1.239 (1) | 0.025 (1) |
| Cuvierian tubules | 11.359 (1) | 0.278 (1) | |
| Bohadschia subrubra | Body wall | 1.789 (1) | 0.064 (1) |
| Cuvierian tubules | 4.724 (1) | 0.094 (1) | |
| Holothuria atra | Body wall | 0.973 ± 1.846 (4) | 0.040 ± 0.045 (4) |
| Holothuria leucospilota | Body wall | 0.324 ± 0.173 (5) | 0.039 ± 0.032 (5) |
| Cuvierian tubules | 1.377 ± 0.864 (5) | 0.040 ± 0.053 (5) | |
| Pearsonothuria graeffei | Body wall | 2.404 ± 0.506 (4) | 0.026 ± 0.012 (4) |
| Cuvierian tubules | 5.361 ± 6.759 (5) | 0.189 ± 0.177 (4) |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Van Dyck, S.; Gerbaux, P.; Flammang, P. Qualitative and Quantitative Saponin Contents in Five Sea Cucumbers from the Indian Ocean. Mar. Drugs 2010, 8, 173-189. https://doi.org/10.3390/md8010173
Van Dyck S, Gerbaux P, Flammang P. Qualitative and Quantitative Saponin Contents in Five Sea Cucumbers from the Indian Ocean. Marine Drugs. 2010; 8(1):173-189. https://doi.org/10.3390/md8010173
Chicago/Turabian StyleVan Dyck, Séverine, Pascal Gerbaux, and Patrick Flammang. 2010. "Qualitative and Quantitative Saponin Contents in Five Sea Cucumbers from the Indian Ocean" Marine Drugs 8, no. 1: 173-189. https://doi.org/10.3390/md8010173
APA StyleVan Dyck, S., Gerbaux, P., & Flammang, P. (2010). Qualitative and Quantitative Saponin Contents in Five Sea Cucumbers from the Indian Ocean. Marine Drugs, 8(1), 173-189. https://doi.org/10.3390/md8010173