The Contribution of Fluoride to the Pathogenesis of Eye Diseases: Molecular Mechanisms and Implications for Public Health
Abstract
:1. Introduction
2. The Role of Fluoride in Oral Health and Dietary Sources of Fluoride
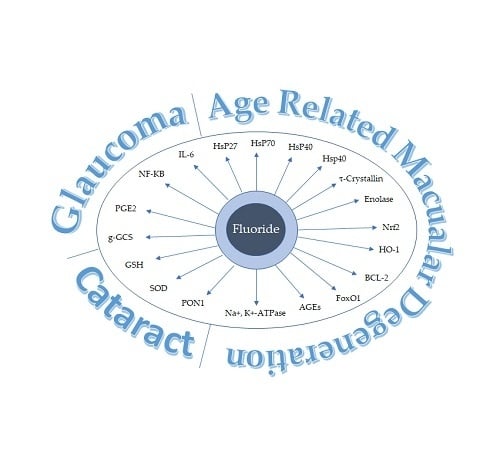
3. Molecular and Biochemical Markers Relevant to the Pathophysiology of Eye Diseases
3.1. The Role of Oxidative Stress and Antioxidants in Eye Disease
3.2. The Role of Na+, K+-ATPase Activity in Degenerative Eye Diseases
3.3. Nuclear Factor Erythroid-2-Related Factor 2 Nuclear Factor
3.4. Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells (NF-kB)
3.5. B-Cell Lymphoma 2 (BCL-2)
3.6. Forkhead Box Protein FoxO Proteins
3.7. Interleukin 6
3.8. Paraoxonase 1
4. Molecular Mechanisms Underlying Fluoride Contribution to Eye Diseases
4.1. Fluoride Inhibition of Carbohydrate Metabolism
4.2. Fluoride and Heat Shock Proteins
4.3. Fluoride Inhibition of Na+, K+-ATPase Activity
4.4. Fluoride Inhibition of Nrf2
4.5. Fluoride Activation of NF-Kb Expression
4.6. Fluoride Downregulates BCL-2, FoxO1 mRNA and Protein Activity and Upregulates IL-6 mRNA Expression and Activity
4.7. Fluoride Inhibits Anitoxidant Activity Including SOD and PON1 Activity
4.7.1. Fluoride Inhibits PON1 Activity
4.7.2. Fluoride Inhibits Glutathione
5. Discussion
6. Additional Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, G.F.; Zou, X.L. Tissue factor with age-related macular degeneration. Int. J. Ophthalmol. 2012, 5, 609–613. [Google Scholar] [PubMed]
- Cai, L.; Liao, H.F.; Zhang, X.J.; Shao, Y.; Xu, M.; Yi, J.L. Acetylcholinesterase function in apoptotic retina pigment epithelial cells induced by H2O2. Int. J. Ophthalmol. 2013, 6, 772–777. [Google Scholar] [PubMed]
- Chiu, C.J.; Chang, M.L.; Zhang, F.F.; Li, T.; Gensler, G.; Schleicher, M.; Taylor, A. The relationship of major American dietary patterns to age-related macular degeneration. Am. J. Ophthalmol. 2014, 158, 118–127.e1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Y.; Tie, L.J.; Wu, S.S.; Lv, P.L.; Huang, H.W.; Wang, W.Q.; Wang, H.; Ma, L. Overweight, Obesity, and Risk of Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Hyman, L.; Schachat, A.P.; He, Q.; Leske, M.C. Hypertension, cardiovascular disease, and age-related macular degeneration. Age-Related Macular Degeneration Risk Factors Study Group. Arch. Ophthalmol. 2000, 118, 351–358. [Google Scholar] [CrossRef]
- Tan, J.S.; Mitchell, P.; Smith, W.; Wang, J.J. Cardiovascular risk factors and the long-term incidence of age-related macular degeneration: The Blue Mountains Eye Study. Ophthalmology 2007, 114, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Tomany, S.C.; Wang, J.J.; Van Leeuwen, R.; Klein, R.; Mitchell, P.; Vingerling, J.R.; Klein, B.E.; Smith, W.; De Jong, P.T. Risk factors for incident age-related macular degeneration: Pooled findings from 3 continents. Ophthalmology 2004, 111, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Rong, S.S.; Xu, Q.; Tang, F.Y.; Liu, Y.; Gu, H.; Tam, P.O.; Chen, L.J.; Brelén, M.E.; Pang, C.P.; et al. Diabetes mellitus and risk of age-related macular degeneration: A systematic review and meta-analysis. PLoS ONE 2014, 9, e108196. [Google Scholar] [CrossRef] [PubMed]
- He, M.S.; Chang, F.L.; Lin, H.Z.; Wu, J.L.; Hsieh, T.C.; Lee, Y.C. The Association Between Diabetes and Age-Related Macular Degeneration Among the Elderly in Taiwan. Diabetes Care 2018, 41, 2202–2211. [Google Scholar] [CrossRef]
- Evans, J.R. Risk factors for age-related macular degeneration. Prog. Retin. Eye Res. 2001, 20, 227–253. [Google Scholar] [CrossRef]
- Babizhayev, M.A.; Yegorov, Y.E. Reactive Oxygen Species and the Aging Eye: Specific Role of Metabolically Active Mitochondria in Maintaining Lens Function and in the Initiation of the Oxidation-Induced Maturity Onset Cataract—A Novel Platform of Mitochondria-Targeted Antioxidants with Broad Therapeutic Potential for Redox Regulation and Detoxification of Oxidants in Eye Diseases. Am. J. Ther. 2016, 23, e98–e117. [Google Scholar] [PubMed]
- Prokofyeva, E.; Wegener, A.; Zrenner, E. Cataract prevalence and prevention in Europe: A literature review. Acta Ophthalmol. 2013, 91, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Tavani, A.; Negri, E.; La Vecchia, C. Food and nutrient intake and risk of cataract. Ann. Epidemiol. 1996, 6, 41–46. [Google Scholar] [CrossRef]
- Abraham, A.G.; Condon, N.G.; West Gower, E. The new epidemiology of cataract. Ophthalmol. Clin. N. Am. 2006, 19, 415–425. [Google Scholar]
- Trautner, C.; Haastert, B.; Richter, B.; Berger, M.; Giani, G. Incidence of Blindness in Southern Germany Due to Glaucoma and Degenerative Conditions. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1031–1034. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Lyu, D.; Dong, X.; He, J.; Yao, K. Hypertension and risk of cataract: A meta-analysis. PLoS ONE 2014, 9, e114012. [Google Scholar] [CrossRef] [PubMed]
- Gnad, H.D.; Rett, A. Ophthalmologische symptome beim Down syndrom. Wien. Klin. Wochenschr. 1979, 91, 735–737. [Google Scholar] [PubMed]
- Rochels, R.; Nover, A.; Schmid, F. Ophthalmologische symptome beim Mongolismussyndrom. Albrecht Von Graefes. Arch. Klin. Exp. Ophthalmol. 1977, 205, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, R.P.; Moreira, J.B. Ocular findings in Down’s syndrome. Am. J. Ophthalmol. 1996, 122, 236–244. [Google Scholar] [CrossRef]
- Puri, B.K.; Singh, I. Prevalence of cataract in adult Down’s syndrome patients aged 28 to 83 years. Clin. Pract. Epidemiol. Ment. Health 2007, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Ruigomez, A.; Garcia Rodriguez, L.A.; Dev, V.J.; Arellano, F.; Raniwala, J. Are schizophrenia or antipsychotic drugs a risk factor for cataracts? Epidemiology 2000, 11, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wan, X.H.; Zhao, G.H. Meta-analysis of the risk of cataract in type 2 diabetes. BMC Ophthalmol. 2014, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Khairallah, M.; Kahloun, R.; Bourne, R.; Limburg, H.; Flaxman, S.R.; Jonas, J.B.; Keeffe, J.; Leasher, J.; Naidoo, K.; Pesudovs, K.; et al. Number of People Blind or Visually Impaired by Cataract Worldwide and in World Regions, 1990 to 2010. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6762–6769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMonnies, C.W. Glaucoma history and risk factors. J. Optom. 2016, 10, 71–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirooka, K.; Shiraga, F. Potential role for angiotensin-converting enzyme inhibitors in the treatment of glaucoma. Clin. Ophthalmol. 2007, 1, 217–223. [Google Scholar]
- Deloitte Access Economics. The Economic Impact of Vision Impairment and Blindness in the Republic of Ireland NCBI (National Council for the Blind of Ireland) May 2011. Available online: http://www.eyedoctors.ie/documents/Cost_of_Sight_Loss_Full_Repor.pdf (accessed on 9 January 2019).
- Eurostat. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=File:Surgical_operations_and_procedures_performed_in_hospitals_%E2%80%94_top_10_procedures_group_1,_2015_or_2016_(per_100_000_inhabitants)_HLTH18.png (accessed on 9 January 2019).
- Akuffo, K.O.; Nolan, J.; Stack, J.; Moran, R.; Feeney, J.; Kenny, R.A.; Peto, T.; Dooley, C.; O’Halloran, A.M.; Cronin, H.; et al. Prevalence of age-related macular degeneration in the Republic of Ireland. Br. J. Ophthalmol. 2015, 99, 1037–1044. [Google Scholar] [CrossRef] [Green Version]
- Nolan, J.; Kenny, R.; O’Regan, C.; Cronin, H.; Loughman, J.; Conolly, E.; Kearney, P.; Beatty, S. Macular Pigment Optical Density in an Aging Irish Population: The Irish Longitudinal Study on Ageing. Ophthalm. Res. 2010, 44, 131–139. [Google Scholar] [CrossRef]
- Augood, C.A.; Vingerling, J.R.; de Jong, P.T.; Chakravarthy, U.; Seland, J.; Soubrane, G.; Tomazzoli, L.; Topouzis, F.; Bentham, G.; Rahu, M.; et al. Prevalence of age-related maculopathy in older Europeans: The European Eye Study (EUREYE). Arch. Ophthalmol. 2006, 124, 529–535. [Google Scholar] [CrossRef]
- Colijn, J.M.; Buitendijk, G.H.S.; Prokofyeva, E.; Alves, D.; Cachulo, M.L.; Khawaja, A.P.; Cougnard-Gregoire, A.; Merle, B.M.J.; Korb, C.; Erke, M.G.; et al. Prevalence of Age-Related Macular Degeneration in Europe: The Past and the Future. Ophthalmology 2017, 124, 1753–1763. [Google Scholar] [CrossRef]
- Owen, C.G.; Jarrar, Z.; Wormald, R.; Cook, D.G.; Fletcher, A.E.; Rudnicka, A.R. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br. J. Ophthalmol. 2012, 96, 752–756. [Google Scholar] [CrossRef] [Green Version]
- Jonasson, F.; Arnarsson, A.; Sasaki, H.; Peto, T.; Sasaki, K.; Bird, A.C. The prevalence of age-related maculopathy in iceland: Reykjavik eye study. Arch. Ophthalmol. 2003, 121, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Björnsson, O.M.; Syrdalen, P.; Bird, A.C.; Peto, T.; Kinge, B. The prevalence of age-related maculopathy (ARM) in an urban Norwegian population: The Oslo Macular study. Acta Ophthalmol. Scand. 2006, 84, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.K.; Cruickshanks, K.J. The prevalence of age-related maculopathy by geographic region and ethnicity. Prog. Retin. Eye Res. 1999, 18, 371–389. [Google Scholar] [CrossRef]
- Kawasaki, R.; Wang, J.J.; Ji, G.J.; Taylor, B.; Oizumi, T.; Daimon, M.; Kato, T.; Kawata, S.; Kayama, T.; Tano, Y.; et al. Prevalence and risk factors for agerelated macular degeneration in an adult Japanese population: The Funagata study. Ophthalmology 2008, 115, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Chou, C.F.; Klein, B.E.; Zhang, X.; Meuer, S.M.; Saaddine, J.B. Prevalence of age-related macular degeneration in the US population. Arch. Ophthalmol. 2011, 129, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Rowland, M.L.; Harris, M.I. Racial/ethnic differences in age-related maculopathy: Third National Health and Nutrition Survey. Ophthalmology 1995, 102, 371–381. [Google Scholar] [CrossRef]
- Congdon, N.; Vingerling, J.R.; Klein, B.E.; West, S.; Friedman, D.S.; Kempen, J.; O’Colmain, B.; Wu, S.Y.; Taylor, H.R.; Eye Diseases Prevalence Research Group. Prevalence of Cataract and Pseudophakia/Aphakia Among Adults in the United States. Arch. Ophthalmol. 2004, 122, 487–494. [Google Scholar]
- Lindstrom, R. Thoughts on cataract surgery. Rev. Ophthalmol. 2015. Available online: https://www.reviewofophthalmology.com/article/thoughts-on--cataract-surgery-2015 (accessed on 10 January 2019).
- Busbee, B.G.; Brown, M.M.; Brown, G.C.; Sharma, S. Incremental cost-effectiveness of initial cataract surgery. Ophthalmology 2002, 109, 606–612. [Google Scholar] [CrossRef]
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013, 36, 1033–1046. [Google Scholar] [CrossRef]
- Keel, S.; Xie, J.; Foreman, J.; van Wijngaarden, P.; Taylor, H.R.; Dirani, M. Prevalence of Age-Related Macular Degeneration in Australia: The Australian National Eye Health Survey. JAMA Ophthalmol. 2017, 135, 1242–1249. [Google Scholar] [CrossRef]
- Keeffe, J.E.; Taylor, H.R. Cataract surgery in Australia 1985–1994. Aust. N. Z. J. Ophthalmol. 1996, 24, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.G.; Wang, J.J.; Rochtchina, E.; Mitchell, P. Comparison of age-specific cataract prevalence in two population-based surveys 6 years apart. BMC Ophthalmol. 2006, 6, 17. [Google Scholar] [CrossRef] [PubMed]
- McCarty, C.A.; Mukesh, B.N.; Fu, C.L.; Taylor, H.R. The epidemiology of cataract in Australia. Am. J. Ophthalmol. 1999, 128, 446–465. [Google Scholar] [CrossRef]
- Rochtchina, E.; Mukesh, B.N.; Wang, J.J.; McCarty, C.A.; Taylor, H.R.; Mitchell, P. Projected prevalence of age-related cataract and cataract surgery in Australia for the years 2001 and 2021: Pooled data from two population-based surveys. Clin. Exp. Ophthalmol. 2003, 31, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Worsley, D.; Worsley, A. Prevalence predictions for age-related macular degeneration in New Zealand have implications for provision of healthcare services. N. Z. Med. J. 2015, 128, 44–55. [Google Scholar] [PubMed]
- Access Economics. Clear focus—The Economic Impact of Vision Loss in New Zealand in 2009. A Report for Vision 2020 Australia in Support of the Vision 2020 New Zealand Trust; Access Economics Pty Limited: Melbourne, Australia, 2010; Available online: http://blindfoundation.org.nz/learn/blindness/clear-focus (accessed on 10 January 2019).
- National Health Committee. Age-Related Macular Degeneration, Tier 2 Assessment Consultation Submissions; National Health Committee: Wellington, New Zealand, 2015. Available online: http://nhc.health.govt.nz (accessed on 10 January 2019).
- Blind Foundation. Eye Diseases. Available online: https://blindfoundation.org.nz/eye-info/latest-statistics/ (accessed on 29 January 2019).
- Sorsby, A.; Harding, R. Experimental degeneration of the retina. Br. J. Ophthalmol. 1960, 44, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Sorsby, A.; Harding, R. Oxidizing agents as potentiators of the retinotoxic action of sodium fluoride, sodium iodate and sodium iodoacetate. Nature 1966, 210, 997–998. [Google Scholar] [CrossRef] [PubMed]
- Orzalesi, N.; Grignolo, A.; Calabria, A. Experimental degeneration of the rabbit retina induced by sodium fluoride. Exp. Eye Res. 1967, 6, 165–170. [Google Scholar] [CrossRef]
- Orzalesi, N.; Grignolo, A.; Calabria, G.A.; Castellazzo, R. A study on the fine structure and the rhodopsin cycle of the rabbit retina in experimental degeneration induced by diaminodiphenoxypentane. Exp. Eye Res. 1967, 6, 376–382. [Google Scholar] [CrossRef]
- Vanysek, J.; Anton, M.; Hrachovina, V.; Moster, M. Some metabolic disturbances of the retina due to the effect of natrium fluoride. Ophthalmologica 1969, 158, 684–690. [Google Scholar]
- Shukla, N.; Pandey, G.S. Fluoride level in cataract lenses in an urban area of India. Fluoride 1991, 24, 40–43. [Google Scholar]
- Rapaport, I. Les opacifications du cristallin mongolisme et cataracte sénile (Donées statisques récentes). Rev. Anthropol. (Paris) 1957, 2, 133–135. [Google Scholar]
- Rapaport, I. Contribution a l’étude etiologique du mongolisme: Rôle des inhibiteurs enzymatiques. L’Encéphale 1957, 46, 468–481. [Google Scholar] [PubMed]
- Rapaport, I. Oligophrénie mongolienne et ectodermoses congénitales. Ann. Dermatol. Syphiligr. 1960, 87, 263–278. [Google Scholar]
- Kas’ianenko, A.S.; Korneva, T.S.; Kovgan, N.I. Dissemination of senile cataract among the population of Poltava Province consuming water with various fluorine levels. Oftalmol. Zh. 1984, 5, 302–304. [Google Scholar]
- Aytuluner, E.; Mensiz, E. Heavy iridocorneal angle hyperpigmentation and glaucoma associated with fluorosis. J. Toxicol. Cutaneous Ocular Toxicol. 2002, 21, 203–212. [Google Scholar] [CrossRef]
- Tomar, S.; Sharma, A.; Tripathi, S. Fluoride intake increases oxidative burden of cataractogenesis in fluoride endemic areas in India, Abstract. In Proceedings of the XXXII Congress of the ESCRS, London, UK, 13–17 September 2014. [Google Scholar]
- Aytuluner, E.; Mensiz, E.; Candir, O.; Aydin, S. Cataractogenic effect of fluorosis in an animal model. J. Toxicol. Cutaneous Ocular Toxicol. 2003, 22, 23–31. [Google Scholar] [CrossRef]
- Mishra, S.; Tomar, S.; Sharma, A.; Chauhan, D.S.; Tripathi, S. Fluoride Induces Morphological and Biochemical Changes in Goat Eye Lens. J. Environ. Anal. Toxicol. 2014, 4, 231. [Google Scholar] [CrossRef]
- Nordmann, J.; Mandel, P.; Archard, M. Inhibition of sugar metabolism in the lens. Br. J. Ophthal. 1954, 38, 673. [Google Scholar] [CrossRef]
- Ashton, N.; Graymore, C.; Petlar, C. Studies on developing retinal vessels. Br. J. Ophthalmol. 1957, 41, 449–460. [Google Scholar] [CrossRef]
- Graymore, C. In vitro swelling of the kitten retina induced by sodium fluoride inhibition. Br. J. Ophthalmol. 1959, 43, 40–41. [Google Scholar] [CrossRef] [PubMed]
- Kleifeld, O.; Hockwine, O.; Ayberk, N. The effect of sodium fluoride on the metabolism of the lens. Graefes Arch. Ophthalmol. 1956, 158, 39–46. [Google Scholar] [CrossRef]
- Dickens, F.; Simer, F. Observations on Tissue Glycolysis: The effect of fluoride and some other substances. Biochem. J. 1929, 23, 936–958. [Google Scholar] [CrossRef] [PubMed]
- Tomar, S. Fluoride intake increases oxidative burden of cataractognesis in fluoride endemic areas in India. J. Clin. Exp. Ophthalmol. 2014, 5, 119. [Google Scholar]
- Recommendations for using fluoride to prevent and control dental caries in the United States. MMWR 2001, 50, 1–42.
- Chan, L.; Mehra, A.; Saikat, S.; Lynch, P. Human exposure assessment of fluoride from tea (Camellia sinensis L.). Food Res. Int. 2013, 51, 564–570. [Google Scholar] [CrossRef]
- Waugh, D.T.; Potter, W.; Limeback, H.; Godfrey, M. Risk assessment of fluoride intake from tea in the republic of Ireland and its implications for public health and water fluoridation. Int. J. Environ. Res. Public Health 2016, 13, 259. [Google Scholar] [CrossRef] [PubMed]
- Waugh, D.T.; Godfrey, M.; Limeback, H.; Potter, W. Black Tea Source, Production, and Consumption: Assessment of Health Risks of Fluoride Intake in New Zealand. J. Environ. Public Health 2017, 2017, 5120504. [Google Scholar] [CrossRef] [PubMed]
- Scientific Opinion on Dietary Reference Values for fluoride. EFSA J. 2013, 11, 3332. [CrossRef] [Green Version]
- Water Fluoridation for the Prevention of Dental Caries (Review). The Cochrane Library 2015, Issue 6. Available online: https://www.cochrane.org/CD010856/ORAL_water-fluoridation-prevent-tooth-decay (accessed on 11 January 2019).
- Opinion of the Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers Concerning the Safety of Fluorine Compounds in Oral Hygiene Products for Children Under the Age of 6 Years. June 2003. SCCNFP/0653/03. Available online: https://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_024.pdf (accessed on 11 January 2019).
- Liu, X.F.; Zhou, D.D.; Xie, T.; Hao, J.L.; Malik, T.H.; Lu, C.B.; Qi, J.; Pant, O.P.; Lu, C.W. The Nrf2 Signaling in Retinal Ganglion Cells under Oxidative Stress in Ocular Neurodegenerative Diseases. Int. J. Biol. Sci. 2018, 14, 1090–1098. [Google Scholar] [CrossRef]
- Dröge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Grune, T.; Sommerburg, O.; Siems, W.G. Oxidative stress in anemia. Clin. Nephrol. 2000, 53 (Suppl. 1), S18–S22. [Google Scholar] [PubMed]
- Spector, A. Oxidative stress-induced cataract: Mechanism of action. FASEB J. 1995, 9, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, J.A.; Murphy, C.G.; Polansky, J.R.; Juster, R. Age-related changes in human trabecular meshwork cellularity. Investig. Ophthalmol. Vis. Sci. 1981, 21, 714–727. [Google Scholar]
- Kahn, M.G.; Giblin, F.J.; Epstein, D.L. Glutathione in calf trabecular meshwork and its relation to aqueous humor outflow facility. Investig. Ophthalmol. Vis. Sci. 1983, 24, 1283–1287. [Google Scholar]
- Young, R.W. Solar radiation and age-related macular degeneration. Surv. Ophthalmol. 1988, 32, 252–269. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Kern, T.S.; Engerman, R.L.; Armstrong, D. Abnormalities of retinal metabolism in diabetes or experimental galactosemia. III. Effects of antioxidants. Diabetes 1996, 45, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.A.; Wu, G.S. Free radical mediated photoreceptor damage in uveitis. Prog. Retin. Eye Res. 2000, 19, 41–68. [Google Scholar] [CrossRef]
- Hedge, K.R.; Varma, S.D. Prevention of cataract by pyruvate in experimentally diabetic mice. Mol. Cell Biochem. 2005, 269, 115–120. [Google Scholar]
- Hegde, K.R.; Kovtun, S.; Varma, S.D. Inhibition of glycolysis in the retina by oxidative stress: Prevention by pyruvate. Mol. Cell. Biochem. 2010, 343, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, V.M.; Beyer, E.C. Oxidative stress, lens gap junctions, and cataracts. Antioxid. Redox Signal. 2009, 11, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Izzotti, A.; Bagnis, A.; Saccà, S.C. The role of oxidative stress in glaucoma. Mutat. Res. 2006, 612, 105–114. [Google Scholar] [CrossRef]
- McMonnies, C.W. Reactive oxygen species, oxidative stress, glaucoma and hyperbaric oxygen therapy. J. Optom. 2017, 11, 3–9. [Google Scholar] [CrossRef]
- Babizhayev, M.A.; Deyev, A.I.; Yermakova, V.N.; Brikman, I.V.; Bours, J. Lipid peroxidation and cataracts: N-acetylcarnosine as a therapeutic tool to manage age-related cataracts in human and in canine eyes. Drugs R&D 2004, 5, 125–139. [Google Scholar]
- Brennan, L.A.; Kantorow, M. Mitochondrial function and redox control in the aging eye: Role of MsrA and other repair systems in cataract and macular degenerations. Exp. Eye Res. 2009, 88, 195–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottonello, S.; Foroni, C.; Carta, A.; Petrucco, S.; Maraini, G. Oxidative stress and age-related cataract. Ophthalmologica. 2000, 214, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Beswick, H.T.; Harding, J.J. Conformational changes induced in bovine lens alpha-crystallin by carbamylation. Relevance to cataract. Biochem. J. 1984, 223, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011, 15, 1583–1606. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.N. Glutathione and its function in the lens-an overview. Exp. Eye Res. 1990, 50, 771–778. [Google Scholar] [CrossRef]
- Rhee, S.G.; Kang, S.W.; Chang, T.S.; Jeong, W.; Kim, K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life 2001, 52, 35–41. [Google Scholar] [CrossRef]
- Spector, A.; Ma, W.; Wang, R.R.; Kleiman, N.J. Microperoxidases catalytically degrade reactive oxygen species and may be anti-cataract agents. Exp. Eye Res. 1997, 65, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2008, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, S.G.; Boulton, M.E. Consequences of oxidative stress in age-related macular degeneration. Mol. Aspects Med. 2012, 33, 399–417. [Google Scholar] [CrossRef] [Green Version]
- Maurya, O.P.; Mohanty, L.; Bhaduri, G.; Chandra, A. Role of anti-oxidant enzymes superoxide dismutase and catalase in the development of cataract: Study of serum levels in patients with senile and diabetic cataracts. J. Indian Med. Assoc. 2006, 104, 396–397. [Google Scholar]
- Ozmen, B.; Ozmen, D.; Erkin, E.; Güner, I.; Habif, S.; Bayindir, O. Lens superoxide dismutase and catalase activities in diabetic cataract. Clin. Biochem. 2002, 35, 69–72. [Google Scholar] [CrossRef]
- Babizhayev, M.A.; Deyev, A.I.; Linberg, L.F. Lipid peroxidation as a possible cause of cataract. Mech. Age Dev. 1988, 44, 68–89. [Google Scholar] [CrossRef]
- Obara, Y. The oxidative stress in the cataract formation. Nippon-Ganka-Gakkai-Zasshi 1995, 99, 1303–1341. [Google Scholar]
- Yildirim, Z.; Ucgun, N.I.; Yildirim, F. The role of oxidative stress and antioxidants in the pathogenesis of age-related macular degeneration. Clinics 2011, 66, 743–746. [Google Scholar]
- Shen, X.L.; Jia, J.H.; Zhao, P.; Fan, R.; Pan, X.Y.; Yang, H.M.; Liu, L. Changes in blood oxidative and antioxidant parameters in a group of Chinese patients with age-related macular degeneration. J. Nutr. Health Aging 2012, 16, 201–204. [Google Scholar] [CrossRef]
- Jia, L.; Dong, Y.; Yang, H.; Pan, X.; Fan, R.; Zhai, L. Serum superoxide dismutase and malondialdehyde levels in a group of Chinese patients with age-related macular degeneration. Aging Clin. Exp. Res. 2011, 23, 264–267. [Google Scholar] [CrossRef]
- Anand, A.; Sharma, N.K.; Gupta, A.; Prabhakar, S.; Sharma, S.K.; Singh, R. Superoxide dismutase1 levels in North Indian population with age-related macular degeneration. Oxid. Med. Cell. Longev. 2013, 2013, 365046. [Google Scholar] [CrossRef] [PubMed]
- Thangapandiyan, S.; Miltonprabu, S. Epigallocatechin gallate supplementation protects against renal injury induced by fluoride intoxication in rats: Role of Nrf2/HO-1 signaling. Toxicol. Rep. 2014, 1, 12–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, L.R.; Xiao, F.; Yuan, P.; Chen, Y.; Gao, Q.K.; Parnell, L.D.; Meydani, M.; Ordovas, J.M.; Li, D.; Lai, C.Q. Curcumin-supplemented diets increase superoxide dismutase activity and mean lifespan in Drosophila. Age (Dordr.) 2012, 35, 1133–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akinyemi, A.J.; Oboh, G.; Ogunsuyi, O.; Abolaji, A.O.; Udofia, A. Curcumin-supplemented diets improve antioxidant enzymes and alter acetylcholinesterase genes expression level in Drosophila melanogaster model. Metab. Brain Dis. 2018, 33, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Nabavi, S.M.; Abolhasani, F.; Moghaddam, A.H.; Eslami, S. Cytoprotective effects of curcumin on sodium fluoride-induced intoxication in rat erythrocytes. Bull. Environ. Contam. Toxicol. 2012, 88, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; He, F.; Lin, J.F.; Shen, W.; Qiu, Y.W. Tea and Risk of Age-Related Cataracts: A Cross-Sectional Study in Zhejiang Province, China. J. Epidemiol. 2016, 26, 587–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuki, K.; Ozawa, Y.; Yoshida, T.; Kurihara, T.; Hirasawa, M.; Ozeki, N.; Shiba, D.; Noda, K.; Ishida, S.; Tsubota, K. Retinal Ganglion Cell Loss in Superoxide Dismutase 1 Deficiency. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4143–4150. [Google Scholar] [CrossRef] [Green Version]
- Klein, J.A.; Longo-Guess, C.M.; Rossmann, M.P.; Seburn, K.L.; Hurd, R.E.; Frankel, W.N.; Bronson, R.T.; Ackerman, S.L. The harlequin mouse mutation downregulates apoptosis-inducing factor. Nature 2002, 419, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Tezel, G. Oxidative stress in glaucomatous neurodegeneration: Mechanisms and consequences. Prog. Retin. Eye Res. 2006, 25, 490–513. [Google Scholar] [CrossRef] [Green Version]
- Schulz, J.B.; Lindenau, J.; Seyfried, J.; Dichgans, J. Glutathione, oxidative stress and neurodegeneration. Eur. J. Biochem. 2000, 267, 4904–4911. [Google Scholar] [CrossRef] [Green Version]
- Gherghel, D.; Griffiths, H.R.; Hilton, E.J.; Cunliffe, I.A.; Hosking, S.L. Systemic reduction in glutathione levels occurs in patients with primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2005, 46, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Spector, A. Oxidation and cataract. Ciba Found Symp. 1984, 106, 48–64. [Google Scholar] [PubMed]
- Li, Q.; Pogwizd, S.M.; Prabhu, S.D.; Zhou, L. Inhibiting Na+/K+ ATPase can impair mitochondrial energetics and induce abnormal Ca2+ cycling and automaticity in guinea pig cardiomyocytes. PLoS ONE 2014, 9, e93928. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Dasgupta, A.; Banerjee, U.; Chowdhury, P.; Mukhopadhyay, A.; Saha, G.; Singh, O. Role of membrane cholesterol and lipid peroxidation in regulating the Na+/K+-ATPase activity in schizophrenia. Indian J. Psychiatry 2016, 58, 317–325. [Google Scholar] [PubMed] [Green Version]
- Yan, Y.; Haller, S.; Shapiro, A.; Malhotra, N.; Tian, J.; Xie, Z.; Malhotra, D.; Shapiro, J.I.; Liu, J. Ouabain-stimulated trafficking regulation of the Na/K-ATPase and NHE3 in renal proximal tubule cells. Mol. Cell. Biochem. 2012, 367, 175–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delamere, N.A.; Tamiya, S. Expression, regulation and function of Na,K-ATPase in the lens. Prog. Retin. Eye Res. 2004, 23, 593–615. [Google Scholar] [CrossRef] [PubMed]
- Unakar, N.J.; Tsui, J.; Johnson, M. Effect of pretreatment of germanium-1322 on NaC,KC-ATPase and galactose cataracts. Curr. Eye Res. 1997, 16, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Sasaki, H.; Giblin, F.J.; Reddy, V.N. A physiological level of ascorbate inhibits galactose cataract in guinea pigs by decreasing polyol accumulation in the lens epithelium: A dehydroascorbate-linked mechanism. Exp. Eye Res. 1994, 58, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.S.; Tsou, K.C.; Ahmad, S.I.; Rahman, M.A.; Kirmani, T.H. Studies on cataractogenesis in humans and rats with Alloxan-induced diabetes. Ophthalm. Res. 1985, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, G.R.; Chapman, C.J.; Chipault, J.R.; Pfeiffer, D.R. Lipid composition and(NaC C KC)-ATPase activity in rat lens during triparanol-induced cataract formation. Biochim. Biophys. Acta 1981, 644, 1–12. [Google Scholar] [CrossRef]
- Luan, Z.; Reddig, K.; Li, H.S. Loss of Na+/K+-ATPase in Drosophila photoreceptors leads to blindness and age-dependent neurodegeneration. Exp. Neurol. 2014, 261, 791–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Dong, X.; Liu, H.; Chen, X.; Shi, H.; Fan, Y.; Hou, D.; Zhang, X. Astaxanthin protects ARPE-19 cells from oxidative stress via upregulation of Nrf2-regulated phase II enzymes through activation of PI3K/Akt. Mol. Vis. 2013, 19, 1656–1666. [Google Scholar] [PubMed]
- Rushworth, S.A.; Chen, X.L.; Mackman, N.; Ogborne, R.M.; O’Connell, M.A. Lipopolysaccharide-induced heme oxygenase-1 expression in human monocytic cells is mediated via Nrf2 and protein kinase C. J. Immunol. 2005, 175, 4408–4415. [Google Scholar] [CrossRef] [PubMed]
- Pickering, A.M.; Linder, R.A.; Zhang, H.; Forman, H.J.; Davies, K.J. Nrf2-dependent induction of proteasome and Pa28αβ regulator are required for adaptation to oxidative stress. J. Biol. Chem. 2012, 287, 10021–10031. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Hao, J.L.; Xie, T.; Malik, T.H.; Lu, C.B.; Liu, C.; Shu, C.; Lu, C.W.; Zhou, D.D. Nrf2 as a target for prevention of age-related and diabetic cataracts by against oxidative stress. Aging Cell. 2017, 16, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zong, R.; Zhang, Z.; Zhu, C.; Pan, F.; Xiao, X.; Liu, Z.; He, H.; Ma, J.X.; Liu, Z.; et al. SERPINA3K protects against oxidative stress via modulating ROS generation/degradation and KEAP1-NRF2 pathway in the corneal epithelium. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5033–5043. [Google Scholar] [CrossRef] [PubMed]
- Lambros, M.L.; Plafker, S.M. Oxidative Stress and the Nrf2 Anti-Oxidant Transcription Factor in Age-Related Macular Degeneration. Adv. Exp. Med. Biol. 2016, 854, 67–72. [Google Scholar]
- Cao, Y.; Wang, L.; Zhao, J.; Zhang, H.; Tian, Y.; Liang, H.; Ma, Q. Serum Response Factor Protects Retinal Ganglion Cells Against High-Glucose Damage. J. Mol. Neurosci. 2016, 59, 232–240. [Google Scholar] [CrossRef]
- Cho, H.; Hartsock, M.J.; Xu, Z.; He, M.; Duh, E.J. Monomethyl fumarate promotes Nrf2-dependent neuroprotection in retinal ischemia-reperfusion. J. Neuroinflamm. 2015, 12, 239. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, Y.; Wang, J.; Sternberg, P.; Freeman, M.L.; Grossniklaus, H.E.; Cai, J. Age-Related Retinopathy in NRF2-Deficient Mice. PLoS ONE 2011, 6, e19456. [Google Scholar] [CrossRef]
- Jobin, C.; Bradham, C.A.; Russo, M.P.; Juma, B.; Narula, A.S.; Brenner, D.A.; Sartor, R.B. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J. Immunol. 1999, 163, 3474–3483. [Google Scholar]
- Baeuerle, P.A.; Henkle, T. Function and activation of NF-κB in the immune system. Annu. Rev. Immunol. 1994, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J.; Karin, M. Nuclear factor-κB, a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 1997, 336, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Pahl, H.L. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef] [PubMed]
- Alexander, G.; Carlsen, H.; Blomhoff, R. Corneal NF-κB activity is necessary for the retention of transparency in the cornea of UV-B-exposed transgenic reporter mice. Exp. Eye Res. 2006, 82, 700–709. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.H.; Ohgami, K.; Shiratori, K.; Koyama, Y.; Yoshida, K.; Kase, S.; Ohno, S. Inhibition of nuclear factor-kappa B activation attenuates hydrogen peroxide-induced cytotoxicity in human lens epithelial cells. Br. J. Ophthalmol. 2006, 91, 369–371. [Google Scholar] [CrossRef] [Green Version]
- Kauppinen, A.; Paterno, J.J.; Blasiak, J.; Salminen, A.; Kaarniranta, K. Inflammation and its role in age-related macular degeneration. Cell. Mol. Life Sci. 2016, 73, 1765–1786. [Google Scholar] [CrossRef] [Green Version]
- Lukiw, W.; Jones, B.; Bhattacharjee, S.; Alexandrov, P.; Dua, P.; Zhao, Y. TREM2 (chr6p21.1) and CFH (chr1q32) regulation by NF-kB-sensitive miRNAs in age-related macular degeneration (AMD) and Alzheimer’s disease (AD). Investig. Ophthalmol. Vis. Sci. 2013, 54, 3652. [Google Scholar]
- Lupien, C.; Horner, P.; Calkins, D. NFkB signaling in retinal glia mediates progressive neural degeneration and vision decline in glaucoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 415. [Google Scholar]
- Ghosh, S.; Shang, P.; Yazdankhah, M.; Bhutto, I.; Hose, S.; Montezuma, S.R.; Luo, T.; Chattopadhyay, S.; Qian, J.; Lutty, G.A.; et al. Activating the AKT2-nuclear factor-κB-lipocalin-2 axis elicits an inflammatory response in age-related macular degeneration. J. Pathol. 2017, 241, 583–588. [Google Scholar] [CrossRef]
- Grimm, S.; Bauer, M.K.A.; Baeuerle, P.A.; Schulze-Osthoff, K. Bcl-2 down-regulates the activity of transcription factor NF-kB induced upon apoptosis. J. Cell. Biol. 1996, 134, 13–23. [Google Scholar] [CrossRef]
- Seidl, R.; Bidmon, B.; Bajo, M.; Yoo, P.C.; Cairns, N.; LaCasse, E.C.; Lubec, G. Evidence for apoptosis in the fetal Down syndrome brain. J. Child Neurol. 2001, 16, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Wolvetang, E.J.; Wilson, T.J.; Sanij, E.; Busciglio, J.; Hatzistavrou, T.; Seth, A.; Hertzog, P.J.; Kola, I. ETS2 overexpression in transgenic models and in Down syndrome predisposes to apoptosis via the p53 pathway. Hum. Mol. Genet. 2003, 12, 247–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zampieri, B.L.; Biselli-Périco, J.M.; de Souza, J.E.; Bürger, M.C.; Silva Júnior, W.A.; Goloni-Bertollo, E.M.; Pavarino, E.C. Altered Expression of Immune-Related Genes in Children with Down Syndrome. PLoS ONE 2014, 9, e107218. [Google Scholar] [CrossRef] [PubMed]
- Fredrik Jarskog, L.; Gilmore, J.H.; Selinger, E.S.; Lieberman, J.A. Cortical Bcl-2 Protein Expression and Apoptotic Regulation in Schizophrenia. Biol. Psychiatry 2000, 48, 641–650. [Google Scholar] [CrossRef]
- Cipollone, F.; Chiarelli, F.; Iezzi, A.; Fazia, M.L.; Cuccurullo, C.; Pini, B.; De Cesare, D.; Torello, M.; Tumini, S.; Cuccurullo, F.; et al. Relationship between reduced BCL-2 expression in circulating mononuclear cells and early nephropathy in type 1 diabetes. Int. J. Immunopathol. Pharmacol. 2005, 18, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Calnan, D.R.; Brunet, A. The FoxO code. Oncogene 2008, 27, 2276–2288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Horst, A.; Burgering, B.M. Stressing the role of FoxO proteins in lifespan and disease. Nat. Rev. Mol. Cell. Biol. 2007, 8, 440–450. [Google Scholar] [CrossRef]
- Kops, G.J.; Dansen, T.B.; Polderman, P.E.; Saarloos, I.; Wirtz, K.W.; Coffer, P.J.; Huang, T.T.; Bos, J.L.; Medema, R.H.; Burgering, B.M.T. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature 2002, 419, 316–321. [Google Scholar] [CrossRef] [Green Version]
- Nemoto, S.; Finkel, T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science 2002, 295, 2450–2452. [Google Scholar] [CrossRef]
- Das, F.; Ghosh-Choudhury, N.; Dey, N.; Bera, A.; Mariappan, M.M.; Kasinath, B.S.; Ghosh Choudhury, G. High Glucose Forces a Positive Feedback Loop Connecting Akt Kinase and FoxO1 Transcription Factor to Activate mTORC1 Kinase for Mesangial Cell Hypertrophy and Matrix Protein Expression. J. Biol. Chem. 2014, 289, 32703–32716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Qiu, J.; Navarro, I.; Gonzalez, P.; Challa, P. FOXO Protein Expression is Down-Regulated With Aging in the Lens. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4590. [Google Scholar]
- Rose-John, S.; Winthrop, K.; Calabrese, L. The role of IL-6 in host defence against infections: Immunobiology and clinical implications. Nat. Rev. Rheumatol. 2017, 13, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, C.; Højfeldt, G.; Hojman, P. The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res. Treat. 2013, 138, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Gabay, C. Interleukin-6 and chronic inflammation. Arthritis Res. Ther. 2006, 8 (Suppl. 2), S3. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lin, H.; Zhong, X.; Liu, Z.; Geng, Y.; Xie, C.; Chen, W. Discrepant expression of cytokines in inflammation- and age-related cataract patients. PLoS ONE 2014, 9, e109647. [Google Scholar] [CrossRef] [PubMed]
- Potvin, S.; Stip, E.; Sepehry, A.A.; Gendron, A.; Bah, R.; Kouassi, E. Inflammatory cytokine alterations in schizophrenia: A systematic quantitative review. Biol. Psychiatry 2008, 63, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Patterson, P.H. Immune involvement in schizophrenia and autism: Etiology, pathology and animal models. Behav. Brain Res. 2009, 204, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Ali, V.; Goodrick, S.; Rawesh, A.; Katz, D.R.; Miles, J.M.; Yudkin, J.S.; Klein, S.; Coppack, S.W. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J. Clin. Endocrinol. Metab. 1997, 82, 4196–4200. [Google Scholar]
- Straub, R.H.; Hense, H.W.; Andus, T.; Scholmerich, J.; Riegger, G.A.; Schunkert, H. Hormone replacement therapy and interrelation between serum interleukin-6 and body mass index in postmenopausal women. J. Clin. Endocrinol. Metab. 2000, 85, 1340–1344. [Google Scholar] [CrossRef]
- Fernandez-Real, J.M.; Vayreda, M.; Richart, C.; Gutierrez, C.; Broch, M.; Vendrell, J.; Ricart, W.J. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J. Clin. Endocrinol. Metab. 2001, 86, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Kado, S.; Nagase, T.; Nagata, N. Circulating levels of interleukin-6, its soluble receptor and interleukin-6/interleukin-6 receptor complexes in patients with type 2 diabetes mellitus. Acta Diabetol. 1999, 36, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Pickup, J.C.; Mattock, M.B.; Chusney, G.D.; Burt, D. NIDDM as a disease of the innate immune system: Association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia 1997, 40, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Sumegová, K.; Nagyová, Z.; Waczulíková, I.; Žitňanová, I.; Ďuračková, Z. Activity of Paraoxonase 1 and Lipid Profile in Healthy Children. Physiol. Res. 2007, 56, 351–357. [Google Scholar]
- Rozenberg, O.; Shiner, M.; Aviram, M.; Hayek, T. Paraoxonase 1 (PON1) attenuates diabetes development in mice through its antioxidative properties. Free Rad. Biol. Med. 2008, 44, 1951–1959. [Google Scholar] [CrossRef]
- Koren-Gluzer, M.; Aviram, M.; Meilin, E.; Hayek, T. The antioxidant HDL-associated paraoxonase-1 (PON1) attenuates diabetes development and stimulates β-cell insulin release. Atherosclerosis 2011, 219, 532–537. [Google Scholar] [CrossRef]
- Baskol, G.; Karakucuk, S.; Oner, A.O.; Baskol, M.; Kocer, D.; Mirza, E.; Saraymen, R.; Ustdal, M. Serum paraoxonase 1 activity and lipid peroxidation levels in patients with age-related macular degeneration. Ophthalmologica 2006, 220, 12–16. [Google Scholar] [CrossRef]
- Ates, O.; Azizi, S.; Alp, H.H.; Kiziltunc, A.; Beydemir, S.; Cinici, E.; Kocer, I.; Baykal, O. Decreased serum paraoxonase 1 activity and increased serum homocysteine and malondialdehyde levels in age-related macular degeneration. Tohoku J. Exp. Med. 2009, 217, 17–22. [Google Scholar] [CrossRef]
- Javadzadeh, A.; Ghorbanihaghjo, A.; Bahreini, E.; Rashtchizadeh, N.; Argani, H.; Alizadeh, S. Serum paraoxonase phenotype distribution in exudative age-related macular degeneration and its relationship to homocysteine and oxidized low-density lipoprotein. Retina 2012, 32, 658–666. [Google Scholar] [CrossRef]
- Hashim, Z.; Zarina, S. Assessment of paraoxonase activity and lipid peroxidation levels in diabetic and senile subjects suffering from cataract. Clin. Biochem. 2007, 40, 705–709. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Lange, M.L. Multifunctional roles of enolase in Alzheimer’s disease brain: Beyond altered glucose metabolism. J. Neurochem. 2009, 111, 915–933. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Christian, W. Isolation and crystallization of enolase. Biochem. Z. 1942, 310, 384–421. [Google Scholar]
- Cimasoni, G. The Inhibition of Enolase by Fluoride in vitro. Caries Res. 1972, 6, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Chai, G.; Brewer, J.M.; Lovelace, L.L.; Lebioda, L. Fluoride inhibition of enolase: Crystal structure and thermodynamics. Biochemistry 2006, 45, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Pietkiewicz, J.; Gamian, A.; Staniszewska, M.; Danielewicz, R. Inhibition of human muscle-specific enolase by methylglyoxal and irreversible formation of advanced glycation end products. J. Enzyme Inhib. Med. Chem. 2009, 24, 356–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chibber, R.; Molinatti, P.A.; Rosatto, N.; Lambourne, B.; Kohner, E.M. Toxic action of advanced glycation end products on cultured retinal capillary pericytes and endothelial cells: Revelance to diabetic retinopathy. Diabetologia 1997, 40, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Duhaiman, A.S. Glycation of human lens proteins from diabetic and nondiabetic senile cataract patients. Glycoconj. J. 1995, 12, 618–621. [Google Scholar] [CrossRef]
- Zhang, K.L.; Lou, D.D.; Guan, Z.Z. Activation of the AGE/RAGE system in the brains of rats and in SH-SY5Y cells exposed to high level of fluoride might connect to oxidative stress. Neurotoxicol. Teratol. 2015, 48, 49–55. [Google Scholar] [CrossRef]
- Wistow, G.J.; Lietman, T.; Williams, L.A.; Stapel, S.O.; De Jong, W.W.; Horwitz, J.; Piatigorsky, J. τ-Crystallin/α-enolase: One gene encodes both an enzyme and a lens structural protein. J. Cell Biol. 1988, 107, 2729–2736. [Google Scholar] [CrossRef]
- Iida, H.; Yahara, I. Yeast heat-shock protein of Mr 48,000 is an isoprotein of enolase. Nature 1985, 315, 688–690. [Google Scholar] [CrossRef]
- Aaronson, R.M.; Graven, K.K.; Tucci, M.; McDonald, R.J.; Farber, H.W. Non-neuronal enolase is an endothelial hypoxic stress protein. J. Biol. Chem. 1995, 270, 27752–27757. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Miller, D.M. Structural analysis of α-enolase. Mapping the functional domains involved in down-regulation of the c-myc protooncogene. J. Biol. Chem. 2000, 275, 5958–5965. [Google Scholar] [CrossRef] [PubMed]
- Piatigorsky, J. Gene Sharing, Lens Crystallins and Speculations on an Eye/Ear Evolutionary Relationship. Integr. Comp. Biol. 2003, 43, 492–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zigler, J.S.; Sinha, D. βA3/A1-crystallin: More than a lens protein. Prog. Retin. Eye Res. 2014, 44, 62–85. [Google Scholar] [CrossRef] [PubMed]
- Urbak, L.; Vorum, H. Heat shock proteins in the human eye. Int. J. Proteom. 2011, 2010, 479571. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, M.; Ireland, M.; Katar, M.; Maisel, H. Heat shock proteins of chicken lens. J. Cell. Biochem. 2001, 82, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Strunnikova, N.; Baffi, J.; Gonzalez, A.; Silk, W.; Cousins, S.W.; Csaky, K.G. Regulated heat shock protein 27 expression in human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2130–2138. [Google Scholar]
- Hoehenwarter, W.; Tang, Y.; Ackermann, R.; Pleissner, K.P.; Schmid, M.; Stein, R.; Zimny-Arndt, U.; Kumar, N.M.; Jungblut, P.R. Identification of proteins that modify cataract of mouse eye lens. Proteomics 2008, 8, 5011–5024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salehi, Z.; Gholaminia, Z.; Panjtanpanah, M.R. Association of HSP70-2 Gene 1267A/G Polymorphism with Cataract Incidence Among Guilan Population. Iran South Med. J. 2017, 19, 931–939. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, J.; Zhang, L.; Xue, D.; Liu, H.; Liu, P. Genetic polymorphisms of HSP70 in age-related cataract. Cell Stress Chaperones. 2013, 18, 703–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayub, H.; Khan, M.I.; Micheal, S.; Akhtar, F.; Ajmal, M.; Shafique, S.; Benish Ali, S.H.; den Hollander, A.I.; Ahmed, A.; Qamar, R. Association of eNOS and HSP70 gene polymorphisms with glaucoma in Pakistani cohorts. Mol. Vis. 2010, 16, 18–25. [Google Scholar] [PubMed]
- Harris, L.L.; Talian, J.C.; Zelenka, P.S. Contrasting patterns of c-myc and N-myc expression in proliferating, quiescent, and differentiating cells of the embryonic chicken lens. Development 1992, 115, 813–820. [Google Scholar] [PubMed]
- Cavalheiro, G.R.; Matos-Rodrigues, G.E.; Gomes, A.L.; Rodrigues, P.M.G.; Martins, R.A.P. c-myc Regulates Cell Proliferation during Lens Development. PLoS ONE 2014, 9, e87182. [Google Scholar] [CrossRef] [PubMed]
- Ashery-Padan, R.; Marquardt, T.; Zhou, X.; Gruss, P. Pax6 activity in the lens primordium is required for lens formation and for correct placement of a single retina in the eye. Genes Dev. 2000, 14, 2701–2711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panneerselvam, L.; Raghunath, A.; Perumal, E. Differential expression of myocardial heat shock proteins in rats acutely exposed to fluoride. Cell Stress Chaperones. 2017, 22, 743–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Zhao, J.; Wang, J.; Wang, J. Fluoride exposure changed the structure and the expressions of HSP related genes in testes of pubertal rats. Chemosphere 2017, 184, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, A.; Podder, S.; Agarwal, S.; Bhattacharya, S. Fluoride-induced histopathology and synthesis of stress protein in liver and kidney of mice. Arch. Toxicol. 2011, 85, 327–335. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Z.; Xiong, Y.; Xue, W.; Kao, X.; Gao, Y.; Muhammad, N.; Song, D. Selenium increases expression of HSP70 and antioxidant enzymes to lessen oxidative damage in Fincoal-type fluorosis. J. Toxicol. Sci. 2009, 34, 399–405. [Google Scholar] [CrossRef] [Green Version]
- Waugh, D.T. Molecular Mechanisms of Fluoride inhibition of Na+, K+-ATPase activity: Implications for Public Health and Health Inequalities. Int. J. Environ. Res. Public Health 2019, in press. [Google Scholar]
- Opit, L.J.; Potter, H.; Charnock, J.S. The effect of anions on (Na+ + K+)-activated. ATPase. Biochim. Biophys. Acta 1966, 120, 159–161. [Google Scholar] [CrossRef]
- Yoshida, H.; Nagai, K.; Kamei, M.; Nakagawa, Y. Irreversible inactivation of (Na+-K+)-dependent ATPase and K+-dependent phosphatase by fluoride. Biochim. Biophys. Acta 1968, 150, 162–164. [Google Scholar] [CrossRef]
- Millman, M.S.; Omachi, A. The Role of Oxidized Nicotinamide Adenine Dinucleotide in Fluoride Inhibition of Active Sodium Transport in Human Erythrocytes. J. Gen. Physiol. 1972, 60, 337–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, J.D.; Davis, R.L.; Steinberg, M. Fluoride and beryllium interact with the (Na + Kbdependent ATPase as analogs of phosphate. J. Bioenergy Biomembr. 1986, 18, 521–531. [Google Scholar] [CrossRef]
- Murphy, A.J.; Hoover, J.C. Inhibition of the Na/K-ATPase by fluoride. Parallels with its inhibition of the sarcoplasmic reticulum CaATPase. J. Biol. Chem. 1992, 267, 16995–17000. [Google Scholar] [PubMed]
- Façanha, A.R.; de Meis, L. Inhibition of Maize Root H+-ATPase by Fluoride and Fluoroaluminate Complexes. Plant Physiol. 1995, 108, 241–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swann, A.C. Inhibition of (Na+,K+)-ATPase by fluoride: Evidence for a membrane adaptation to ethanol. Alcohol 1990, 7, 91–95. [Google Scholar] [CrossRef]
- Suketa, Y.; Suzuki, K.; Taki, T.; Itoh, Y.; Yamaguchi, M.; Sakurai, T.; Tanishita, Y. Effect of fluoride on the activities of the Na+/glucose cotransporter and Na+/K(+)-ATPase in brush border and basolateral membranes of rat kidney (in vitro and in vivo). Biol. Pharm. Bull. 1995, 18, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Iukhnovets, R.A.; Bachinskiĭ, P.P. Effect of fluoride and insulin on cation-dependent ATPase activity of the enterocytes during threonine absorption. Vopr. Med. Khim. 1982, 28, 46–50. [Google Scholar]
- Zhan, X.A.; Li, J.X.; Wang, M.; Xu, Z.R. Effects of Fluoride on Growth and Thyroid Function in Young Pigs. Fluoride 2006, 39, 95–100. [Google Scholar]
- Sarkar, C.; Pal, S. Ameliorative effect of resveratrol against fluoride-induced alteration of thyroid function in male wistar rats. Biol. Trace Elem. Res. 2014, 162, 278–287. [Google Scholar] [CrossRef]
- Sarkar, C.; Pal, S. Effects of sub-acute fluoride exposure on discrete regions of rat brain associated with thyroid dysfunction: A comparative study. Int. J. Biomed. Res. 2015, 6, 647–660. [Google Scholar] [CrossRef]
- Arulkumar, M.; Vijayan, R.; Penislusshiyan, S.; Sathishkumar, P.; Angayarkanni, J.; Palvannan, T. Alteration of paraoxonase, arylesterase and lactonase activities in people around fluoride endemic area of Tamil Nadu, India. Clin. Chim. Acta 2017, 471, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Shashi, A.; Meenakshi, G. Inhibitory Effect of Fluoride on Na+,K+ ATPase Activity in Human Erythrocyte Membrane. Biol. Trace Elem. Res. 2015, 168, 340–348. [Google Scholar]
- Gallicchio, M.A.; Bach, L.A. Advanced glycation end products inhibit Na+ K+ ATPase in proximal tubule epithelial cells: Role of cytosolic phospholipase A2alpha and phosphatidylinositol 4-phosphate 5-kinase gamma. Biochim. Biophys. Acta 2010, 1803, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Thangapandiyan, S.; Miltonprabu, S. Epigallocatechin gallate exacerbates fluoride-induced oxidative stress mediated testicular toxicity in rats through the activation of Nrf2 signaling pathway. Asian Pacif. J. Reprod. 2015, 4, 272–287. [Google Scholar] [CrossRef]
- Janowiak, B.E.; Hayward, M.A.; Peterson, F.C.; Volkman, B.F.; Griffith, O.W. Gamma-glutamylcysteine synthetase-glutathione synthetase: Domain structure and identification of residues important in substrate and glutathione binding. Biochemistry 2006, 45, 10461–10473. [Google Scholar] [CrossRef] [PubMed]
- Carnicer, M.J.; Bernardini, S.; Bellincampi, L.; Noguera, N.I.; Nuccetelli, M.; Ammatuna, E.; Breccia, M.; Lo-Coco, F.; Federici, G. Role of gamma-glutamyl cysteine synthetase (gamma-GCS) gene expression as marker of drug sensitivity in acute myeloid leukemias. Clin. Chim. Acta 2006, 365, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.; Bellner, L.; Cullaro, G.; Gotlinger, K.H.; Dunn, M.W.; Schwartzman, M.L. Heme oxygenase-1 induction attenuates corneal inflammation and accelerates wound healing after epithelial injury. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3379–3386. [Google Scholar] [CrossRef]
- Nath, D.A.; Balla, G.; Vercelotti, G.M.; Balla, J.; Jacob, H.S.; Levitt, M.D.; Rosenberg, M.E. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J. Clin. Investig. 1992, 90, 267–270. [Google Scholar] [CrossRef]
- Choi, A.M.K.; Alam, J. Heme Oxygenase-1: Function, Regulation, and Implication of a Novel Stress-inducible Protein in Oxidant-induced Lung Injury. Am. J. Respir. Cell. Mol. Biol. 1996, 15, 9–19. [Google Scholar] [CrossRef]
- Seta, F.; Bellner, L.; Rezzani, R.; Regan, R.F.; Dunn, M.W.; Abraham, N.G.; Gronert, K.; Laniado-Schwartzman, M. Heme oxygenase-2 is a critical determinant for execution of an acute inflammatory and reparative response. Am. J. Pathol. 2006, 169, 1612–1623. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Gupta, S.K.; Kumar, K.; Trivedi, R.; Godbole, M.M. Simultaneous exposure of excess fluoride and calcium deficiency alters VDR, CaR, and calbindin D 9 k mRNA levels in rat duodenal mucosa. Calcif. Tissue Int. 2004, 75, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Kong, J.; Duan, Y.; Szeto, F.L.; Liao, A.; Madara, J.L.; Li, Y.C. Increased NF-kappaB activity in fibroblasts lacking the vitamin D receptor. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E315–E322. [Google Scholar] [CrossRef] [PubMed]
- Stachowska, E.; Baśkiewicz-Masiuk, M.; Machaliński, B.; Rybickam, M.; Gutowska, I.; Bober, J.; Grymula, K.; Dziedziejko, V.; Chlubek, D. Sodium fluoride enhancement of monocyte differentiation via nuclear factor κB mechanism. Fluoride 2005, 38, 297–306. [Google Scholar]
- Chen, Q.; Wang, Z.; Xiong, Y.; Zou, X.; Liu, Z. Comparative study of p38 MAPK signal transduction pathway of peripheral blood mononuclear cells from patients with coal-combustion-type fluorosis with and without high hair selenium levels. Int. J. Hyg. Environ. Health 2010, 213, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Huo, M.; Li, G.; Wang, J. Regulation of LPS-induced mRNA expression of pro-inflammatory cytokines via alteration of NF-κB activity in mouse peritoneal macrophages exposed to fluoride. Chemosphere 2016, 161, 89–95. [Google Scholar] [CrossRef]
- Jones, E.; Adcock, I.M.; Ahmed, B.Y.; Punchard, N.A. Modulation of LPS stimulated NF-kappaB mediated Nitric Oxide production by PKCε and JAK2 in RAW macrophages. J. Inflamm. (Lond.) 2007, 4, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Q.; Cui, H.; Deng, H.; Kuang, P.; Liu, H.; Lu, Y.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; et al. Sodium fluoride induces renal inflammatory responses by activating NF-κB signaling pathway and reducing anti-inflammatory cytokine expression in mice. Oncotarget 2017, 8, 80192–80207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Refsnes, M.; Skuland, T.; Låg, M.; Schwarze, P.E.; Øvrevik, J. Differential NF-κB and MAPK activation underlies fluoride- and TPA-mediated CXCL8 (IL-8) induction in lung epithelial cells. J. Inflamm. Res. 2014, 7, 169–185. [Google Scholar] [CrossRef]
- Deng, H.; Kuang, P.; Cui, H.; Lou, Q.; Liu, H.; Lu, Y.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; et al. Sodium fluoride induces apoptosis in mouse splenocytes by activating ROS-dependent NF-κB signalling. Oncotarget 2017, 8, 114428–114441. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, A.; Xia, T.; He, P. Effects of fluoride on DNA damage, S-phase cell-cycle arrest and the expression of NF-kappaB in primary cultured rat hippocampal neurons. Toxicol. Lett. 2008, 179, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhu, W.J.; Xu, X.H.; Zhang, Z.G. Effect of fluoride on calcium ion concentration and expression of nuclear transcription factor kappa-B ρ65 in rat hippocampus. Exp. Toxicol. Pathol. 2011, 63, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Misra, U.K.; Gawdi, G.; Pizzo, S.V. Beryllium fluoride-induced cell proliferation: A process requiring P21(ras)-dependent activated signal transduction and NF-kappaB-dependent gene regulation. J. Leukoc. Biol. 2002, 71, 487–494. [Google Scholar] [PubMed]
- Shanmugam, T.; Abdulla, S.; Yakulasamy, V.; Selvaraj, M.; Mathan, R. A mechanism underlying the neurotoxicity induced by sodium fluoride and its reversal by epigallocatechin gallate in the rat hippocampus: Involvement of NrF2/Keap-1 signaling pathway. J. Basic Appl. Zool. 2018, 79, 1–19. [Google Scholar] [CrossRef]
- Yan, N.; Liu, Y.; Liu, S. Fluoride-Induced Neuron Apoptosis and Expressions of Inflammatory Factors by Activating Microglia in Rat Brain. Mol. Neurobiol. 2016, 53, 4449–4460. [Google Scholar] [CrossRef]
- Sun, Y.; Ke, L.; Zheng, X.; Li, T.; Ouyang, W.; Zhang, Z. Effects of Different Levels of Calcium Intake on Brain Cell Apoptosis in Fluorosis Rat Offspring and Its Molecular Mechanism. Biol. Trace Elem. Res. 2017, 176, 355–366. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z. Effects of chronic fluorosis on CAMKIIα, C-FOS, BAX, and BCL-2 channel signalling in the Hippocampus of Rats. Fluoride 2013, 46, 135–141. [Google Scholar]
- Zhang, W.L.; Cui, Y.N.; Gao, S.; Zhang, X.Y.; Li, G.S. Expression of proto-oncogenes c-fos and c-jun in osteoblasts activated by excessive fluoride. Zhonghua Yu Fang Yi Xue Za Zhi 2003, 37, 246–250. [Google Scholar]
- Teng, Y.; Zhang, J.; Zhang, Z.; Feng, J. The Effect of Chronic Fluorosis on Calcium Ions and CaMKIIα, and c-fos Expression in the Rat Hippocampus. Biol. Trace Elem. Res. 2018, 182, 295–302. [Google Scholar] [CrossRef]
- Poche, R.A.; Sharma, R.; Garcia, M.D.; Wada, A.M.; Nolte, M.J.; Udan, R.S.; Paik, J.H.; DePinho, R.A.; Bartlett, J.D.; Dickinson, M.E. Transcription factor FoxO1 is essential for enamel biomineralization. PLoS ONE 2012, 7, e30357. [Google Scholar]
- Gao, J.; Ruan, J.; Gao, L. Excessive fluoride reduces Foxo1 expression in dental epithelial cells of the rat incisor. Eur. J. Oral Sci. 2014, 122, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, L.; Zhao, X.; Wang, P.; Liu, Y.; Ruan, J. Foxo1 attenuates NaF-induced apoptosis of LS8 cells through the JNK and mitochondrial pathways. Biol. Trace Elem. Res. 2017, 181, 104–111. [Google Scholar] [CrossRef]
- Li, J.; Wang, P.; Gao, J.; Fei, X.; Liu, Y.; Ruan, J. NaF Reduces KLK4 Gene Expression by Decreasing Foxo1 in LS8 Cells. Biol. Trace Elem. Res. 2018, 186, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xia, T.; Zhang, M.; He, W.; He, P.; Chen, X.; Yang, K.; Wang, A. Screening of Environmental Response Genes Related To Dental Fluorosis. Fluoride 2006, 39, 195–201. [Google Scholar]
- Akashi, M.; Loussararian, A.H.; Adelman, D.C.; Saito, M.; Koeffler, H.P. Role of lymphotoxin in expression of interleukin 6 in human fibroblasts. Stimulation and regulation. J. Clin. Investig. 1990, 85, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Refsnes, M.; Becher, R.; Lag, M.; Skuland, T.; Schwarze, P.E. Fluoride-induced interleukin-6 and interleukin-8 synthesis in human epithelial lung cells. Hum. Exp. Toxicol. 1999, 18, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, O.K.; Oyewo, E.B.; Adekunle, A.S.; Adedosu, O.T.; Adedeji, A.L. Oxidative indices correlate with dyslipidemia and pro-inflammatory cytokine levels in fluoride-exposed rats. Arh. Hig. Rada Toksikol. 2013, 64, 521–529. [Google Scholar] [CrossRef]
- Ma, Y.; Niu, R.; Sun, Z.; Wang, J.; Luo, G.; Zhang, J.; Wang, J. Inflammatory responses induced by fluoride and arsenic at toxic concentration in rabbit aorta. Arch. Toxicol. 2012, 86, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, P.E.; Låg, M.; Becher, R.; Thrane, E.V.; Samuelsen, J.T.; Hetland, R.B.; Refsnes, M. Role of signal transduction pathways in lung inflammatory responses. Toxicol. Lett. 2000, 112–113, 165–170. [Google Scholar] [CrossRef]
- Chouhan, S.; Lomash, V.; Flora, S.J.S. Fluoride-induced changes in haem biosynthesis pathway, neurological variables and tissue histopathology of rats. J. Appl. Toxicol. 2010, 30, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, H.S.; Allmann, D.W. The effects of fluoridated water on rat urine and tissue cAMP levels. Arch. Oral Biol. 1982, 27, 107–112. [Google Scholar] [CrossRef]
- Raina, R.; Baba, N.A.; Verma, P.K.; Sultana, M.; Singh, M. Hepatotoxicity Induced by Subchronic Exposure of Fluoride and Chlorpyrifos in Wistar Rats: Mitigating Effect of Ascorbic Acid. Biol. Trace Elem. Res. 2015, 166, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Baba, N.A.; Raina, R.; Verma, P.K.; Sultana, M.; Prawez, S.; Nisara, N.A. Toxic effects of fluoride and chlorpyrifos on antioxidant parameters in rats: Protective effects of vitamins C and E. Fluoride 2013, 46, 73–79. [Google Scholar]
- Chlubek, D.; Grucka-Mamczar, E.; Birkner, E.; Polaniak, R.; Starwiarska-Pieta, B.; Duliban, H. Activity of pancreatic antioxidative enzymes and malondialdehyde concentrations in rats with hyperglycemia caused by fluoride intoxication. J. Trace Elem. Med. Biol. 2003, 17, 57–60. [Google Scholar] [CrossRef]
- Viglino, P.; Rigo, A.; Stevanato, R.; Ranieri, G.A. The binding of fluoride ion to bovine cuprozinc superoxide dismutase as studied by 19F magnetic relaxation. J. Magn. Resonance 1969, 34, 265–274. [Google Scholar] [CrossRef]
- Dooley, D.M.; Jones, T.F.; Karas, J.L.; McGuirl, M.A.; Brown, R.D., III; Koenig, S.H. Azide and fluoride binding to E. coli iron superoxide dismutase as studied by solvent proton magnetic relaxation dispersion. J. Am. Chem. Soc. 1987, 109, 721–725. [Google Scholar]
- Varol, E.; Icli, A.; Aksoy, F.; Bas, H.A.; Sutcu, R.; Ersoy, I.H.; Varol, S.; Ozaydin, M. Evaluation of total oxidative status and total antioxidant capacity in patients with endemic fluorosis. Toxicol. Ind. Health 2013, 29, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Kalyanalakshmi, P.; Vijayabhaskar, M.; Dhananjaya Naidu, M. Lipid peroxidation and antioxidant enzyme status of adult males with skeletal fluorosis in Andhra Pradesh, India. Fluoride 2007, 40, 42–45. [Google Scholar]
- Shanthakumari, D.; Srinivasalu, S.; Subramanian, S. Antioxidant defense systems in red blood cell lysates of men with dental fluorosis living in Tamil Nadu, India. Fluoride 2006, 39, 231–239. [Google Scholar]
- Kumari, D.S.; Rao, P.R. Red cell membrane alterations in human chronic fluoride toxicity. Biochem. Int. 1991, 23, 639–648. [Google Scholar] [PubMed]
- Shivarajashankara, Y.M.; Shivashankara, A.R.; Gopalakrishna, B.P.; Rao, S.H. Oxidative stress in children with endemic skeletal fluorosis. Fluoride 2011, 34, 108–113. [Google Scholar]
- Reddy, G.B.; Khandare, A.L.; Reddy, P.Y.; Rao, G.S.; Balakrishna, N.; Srivalli, I. Antioxidant defense system and lipid peroxidation in patients with skeletal fluorosis and in fluoride-intoxicated rabbits. Toxicol. Sci. 2003, 72, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Gavriliuc, L.; Stepco, E.; Lupan, I.; Sevcenco, N.; Spine, I. Salivary Glutathione-Dependent Enzymes in Patients with Dental Fluorosis Treated by Complex Antioxidant Therapy. Balk J. Stom 2012, 16, 79–83. [Google Scholar]
- Anwar, A.J.; Walker, J.D.; Frier, B.M. Type 1 diabetes mellitus and Down’s syndrome: Prevalence, management and diabetic complications. Diabet. Med. 1998, 15, 160–163. [Google Scholar] [CrossRef]
- Bergholdt, R.; Eising, S.; Nerup, J.; Pociot, F. Increased prevalence of Down’s syndrome in individuals with type 1 diabetes in Denmark: A nationwide population-based study. Diabetologia 2006, 49, 1179–1182. [Google Scholar] [CrossRef]
- Prasher, V.P. Prevalence of psychiatric disorders in adults with Down’s syndrome. Eur. J. Psychiatry 1995, 9, 77–82. [Google Scholar]
- Ekstein, S.; Glick, B.; Weill, M.; Kay, B.; Berger, I. Down’s syndrome and attention-deficit/hyperactivity disorder (ADHD). J. Child Neurol. 2011, 26, 1290–1295. [Google Scholar] [CrossRef]
- Vicari, S.; Pontillo, M.; Armando, M. Neurodevelopmental and psychiatric issues in Down’s syndrome: Assessment and intervention. Psychiatr. Genet. 2013, 23, 95–107. [Google Scholar] [CrossRef]
- Dykens, E.M.; Shah, B.; Davis, B.; Baker, C.; Fife, T.; Fitzpatrick, J. Psychiatric disorders in adolescents and young adults with Down’s syndrome and other intellectual disabilities. J. Neurodev. Disord. 2015, 7, 9. [Google Scholar] [CrossRef]
- Tassé, M.J.; Navas Macho, P.; Havercamp, S.M.; Benson, B.A.; Allain, D.C.; Manickam, K.; Davis, S. Psychiatric Conditions Prevalent Among Adults with Down’s syndrome. J. Pol. Pract. Intellect. Disabil. 2016, 13, 173–180. [Google Scholar] [CrossRef]
- Oxelgren, U.W.; Myrelid, Å.; Annerén, G.; Ekstam, B.; Göransson, C.; Holmbom, A.; Isaksson, A.; Åberg, M.; Gustafsson, J.; Fernell, E. Prevalence of autism and attention-deficit-hyperactivity disorder in Down’s syndrome: A population-based study. Dev. Med. Child Neurol. 2017, 59, 276–283. [Google Scholar] [CrossRef]
- Kohen, D. Diabetes mellitus and schizophrenia: Historical perspective. Br. J. Psychiatry Suppl. 2004, 47, S64–S66. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.; Weiden, P.; Delahanty, J.; Goldberg, R.; Postrado, L.; Lucksted, A.; Lehman, A. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr. Bull. 2000, 26, 903–912. [Google Scholar] [CrossRef] [PubMed]
- De Hert, M.; van Winkel, R.; Van Eyck, D.; Hanssens, L.; Wampers, M.; Scheen, A.; Peuskens, J. Prevalence of diabetes, metabolic syndrome and metabolic abnormalities in schizophrenia over the course of the illness: A cross-sectional study. Clin. Pract. Epidemol. Ment. Health 2006, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Stary, J.M.; Halt, A.R.; Realmuto, G.R. Dysregulation of Reelin and Bcl-2 proteins in autistic cerebellum. J. Autism. Dev. Disord. 2001, 31, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Halt, A.R.; Stary, J.M.; Realmuto, G.M.; Jalali-Mousavi, M. Reduction in anti-apoptotic protein Bcl-2 in autistic cerebellum. Neuroreport 2001, 12, 929–933. [Google Scholar] [CrossRef]
- Araghi-Niknam, M.; Fatemi, S.H. Levels of Bcl-2 and P53 are altered in superior frontal and cerebellar cortices of autistic subjects. Cell. Mol. Neurobiol. 2003, 23, 945–952. [Google Scholar] [CrossRef]
- Mal’tseva, V.A. Eye damage in workers with fluorine intoxication. Vestn. Oftalmol. 1973, 2, 71–74. [Google Scholar]
- Karczewicz, D.; Baranowska-George, T.; Palacz, O.; Tokarz-Sawinska, E.; Stankiewicz, W.; Krzystolik, Z.; Lubinski, W.; Kosmider, K. Evaluation of the visual system in peoplehaving extended contact with fluorine. Klin. Oczna 1989, 91, 9–11. [Google Scholar]
- Liu, I.Y.; White, L.; LaCroix, A.Z. The association of age-related macular degeneration and lens opacities in the aged. Am. J. Public Health 1989, 79, 765–769. [Google Scholar] [CrossRef]
- Lin, C.C.; Lin, C.Y.; Ma, H.Y. Pulmonary function changes and increased Th-2 cytokine expression and nuclear factor kB activation in the lung after sensitization and allergen challenge in brown Norway rats. Immunol. Lett. 2000, 73, 57–64. [Google Scholar] [CrossRef]
- Caramori, G.; Casolari, P.; Adcock, I. Role of Transcription Factors in the Pathogenesis of Asthma and COPD. Cell Commun. Adhes. 2013, 20, 21–40. [Google Scholar] [Green Version]
- Caramori, G.; Adcock, I.M.; Ito, K. Anti-inflammatory inhibitors of IkappaB kinase in asthma and COPD. Curr. Opin. Investig. Drugs 2004, 5, 1141–1147. [Google Scholar] [PubMed]
- Edwards, M.R.; Bartlett, N.W.; Clarke, D.; Birrell, M.; Belvisi, M.; Johnston, S.L. Targeting the NF-kappaB pathway in asthma and chronic obstructive pulmonary disease. Pharmacol. Ther. 2009, 121, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Chung, S.W.; Kim, D.H.; Kim, J.M.; Ha, Y.M.; Kim, Y.H.; No, J.K.; Chung, H.S.; Park, K.Y.; Rhee, S.H.; et al. Modulation of age-related NF-kappaB activation by dietary zingerone via MAPK pathway. Exp. Gerontol. 2010, 45, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Naik, U.S.; Gangadharan, C.; Abbagani, K.; Nagalla, B.; Dasari, N.; Manna, S.K. A study of nuclear transcription factor-kappa B in childhood autism. PLoS ONE 2011, 6, e19488. [Google Scholar] [CrossRef] [PubMed]
- Young, A.M.; Campbell, E.; Lynch, S.; Suckling, J.; Powis, S.J. Aberrant NF-kappaB expression in autism spectrum condition: A mechanism for neuroinflammation. Front. Psychiatry. 2011, 2, 27. [Google Scholar] [CrossRef]
- Abdel-Salam, O.M.E.; Youness, E.R.; Mohammed, N.A.; Elhamed, W.A.A. Nuclear Factor-Kappa B and Other Oxidative Stress Biomarkers in Serum of Autistic Children. Open J. Mol. Integr. Physiol. 2015, 5, 18–27. [Google Scholar] [CrossRef]
- Ghanizadeh, A. Nuclear factor kappa B may increase insight into the management of neuroinflammation and excitotoxicity in autism. Expert Opin. Ther. Targets 2011, 15, 781–783. [Google Scholar] [CrossRef]
- Feng, Y.; Li, X.; Zhou, W.; Lou, D.; Huang, D.; Li, Y.; Kang, Y.; Xiang, Y.; Li, T. Regulation of set gene expression by NF- κB. Mol. Neurobiol. 2016, 54, 4477–4485. [Google Scholar] [CrossRef]
- Lukiw, W.J.; Bazan, N.G. Strong nuclear factor-κB-DNA binding parallels cyclooxygenase-2 gene transcription in aging and in sporadic Alzheimer’s disease superior temporal lobe neocortex. J. Neurosci. Res. 1998, 53, 583–592. [Google Scholar] [CrossRef]
- Boissiere, F.; Hunot, S.; Faucheux, B.; Duyckaerts, C.; Hauw, J.J.; Agid, Y.; Hirsch, E.C. Nuclear translocation of NF-κB in cholinergic neurons of patients with Alzheimer’s disease. Neuroreport 1997, 8, 2849–2852. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Nishimura, T.; Kondo, H.; Ikeda, K.; Hayashi, Y.; McGeer, P.L. Expression of the receptor for macrophage colony stimulating factor by brain microglia and its upregulation in brains of patients with Alzheimer’s disease and amyotrophic lateral sclerosis. Brain Res. 1994, 639, 171–174. [Google Scholar] [CrossRef]
- Hunot, S.; Brugg, B.; Ricard, D.; Michel, P.P.; Muriel, M.P.; Ruberg, M.; Faucheux, B.A.; Agid, Y.; Hirsch, E.C. Nuclear translocation of NF-κB is increased in dopaminergic neurons of patients with Parkinson disease. Proc. Natl. Acad. Sci. USA 1997, 94, 7531–7536. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Hong, J.S. Microglia and inflammation-mediated neurodegeneration: Multiple triggers with a common mechanism. Prog. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonetti, B.; Stegagno, C.; Cannella, B.; Rizzuto, N.; Moretto, G.; Raine, C.S. Activation of NF-κB and c-jun transcription factors in multiple sclerosis lesions. Implications for oligodendrocyte pathology. Am. J. Pathol. 1999, 155, 1433–1438. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Kim, J.J.; Mandelli, L.; Lim, S.; Lim, H.K.; Kwon, O.J.; Pae, C.U.; Serretti, A.; Nimgaonkar, V.L.; Paik, I.H.; Jun, T.Y. Association analysis of heat shock protein 70 gene polymorphisms in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2008, 258, 239–244. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Owczarek, A.; Suchanek, R.; Paul-Samojedny, M.; Fila-Danilow, A.; Borkowska, P.; Kucia, K.; Kowalski, J. Heat shock protein 70 gene polymorphisms are associated with paranoid schizophrenia in the Polish population. Cell Stress Chaperones 2014, 19, 205–215. [Google Scholar] [CrossRef]
- El-Ansary, A.; Al-Ayadhi, L. Neuroinflammation in autism spectrum disorders. J. Neuroinflamm. 2012, 9, 265. [Google Scholar] [CrossRef] [Green Version]
- Aron, Y.; Busson, M.; Polla, B.S.; Dusser, D.; Lockhart, A.; Swierczewski, E.; Favatier, F. Analysis of hsp70 gene polymorphism in allergic asthma. Allergy 1999, 54, 165–170. [Google Scholar] [CrossRef]
- Hou, C.; Zhao, H.; Li, W.; Zhenyu, L.; Dan, Z.; Laiyu, L.; Wancheng, T.; Shao-xi, C.; Fei, Z. Increased heat shock protein 70 levels in induced sputum and plasma correlate with severity of asthma patients. Cell Stress Chaperones 2011, 16, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Redlich, K.; Xu, Q.; Bizan, P.; Groger, M.; Tohidast-Akrad, M.; Kiener, H.; Smolen, J.; Steiner, G. Enhanced expression of heat shock protein 70 (hsp70) and heat shock factor 1 (HSF1) activation in rheumatoid arthritis synovial tissue. Differential regulation of hsp70 expression and hsf1 activation in synovial fibroblasts by proinflammatory cytokines, shear stress, and antiinflammatory drugs. J. Clin. Investig. 1998, 102, 302–311. [Google Scholar] [PubMed]
- Luo, X.; Zuo, X.; Zhang, B.; Song, L.; Wei, X.; Zhou, Y.; Xiao, X. Release of heat shock protein 70 and the effects of extracellular heat shock protein 70 on the production of IL-10 in fibroblast-like synoviocytes. Cell Stress Chaperones 2008, 13, 365–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najafizadeh, S.R.; Ghazizadeh, Z.; Nargesi, A.A.; Mahdavi, M.; Abtahi, S.; Mirmiranpour, H.; Nakhjavani, M. Analysis of serum heat shock protein 70 (HSPA1A) concentrations for diagnosis and disease activity monitoring in patients with rheumatoid arthritis. Cell Stress Chaperones 2015, 20, 537–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, E.H.; Kim, D.J.; Lee, E.Y.; Lee, Y.J.; Lee, E.B.; Song, Y.W. Downregulation of heat shock protein 70 protects rheumatoid arthritis fibroblast-like synoviocytes from nitric oxide-induced apoptosis. Arthritis Res. Ther. 2009, 11, R130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ucisik-Akkaya, E.; Davis, C.F.; Gorodezky, C.; Alaez, C.; Dorak, M.T. HLA complex-linked heat shock protein genes and childhood acute lymphoblastic leukemia susceptibility. Cell Stress Chaperones 2010, 15, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Ciocca, D.R.; Clark, G.M.; Tandon, A.K.; Fuqua, S.A.; Welch, W.J.; McGuire, W.L. Heat shock protein hsp70 in patients with axillary lymph node-negative breast cancer: Prognostic implications. J. Natl. Cancer Inst. 1993, 85, 570–574. [Google Scholar] [CrossRef]
- Hwang, T.S.; Han, H.S.; Choi, H.K.; Lee, Y.J.; Kim, Y.J.; Han, M.Y.; Park, Y.M. Differential, stage-dependent expression of Hsp70, Hsp110 and Bcl-2 in colorectal cancer. J. Gastroenterol. Hepatol. 2003, 18, 690–700. [Google Scholar] [CrossRef]
- Joo, M.; Chi, J.G.; Lee, H. Expressions of HSP70 and HSP27 in hepatocellular carcinoma. J. Korean Med. Sci. 2005, 20, 829–834. [Google Scholar] [CrossRef]
- Luk, J.M.; Lam, C.T.; Siu, A.F.; Lam, B.Y.; Ng, I.O.; Hu, M.Y.; Che, C.M.; Fan, S.T. Proteomic profiling of hepatocellular carcinoma in Chinese cohort reveals heat-shock proteins (Hsp27, Hsp70, GRP78) up-regulation and their associated prognostic values. Proteomics 2006, 6, 1049–1105. [Google Scholar] [CrossRef] [PubMed]
- Alaiya, A.A.; Oppermann, M.; Langridge, J.; Roblick, U.; Egevad, L.; Brindstedt, S.; Hellström, M.; Linder, S.; Bergman, T.; Jörnvall, H.; et al. Identification of proteins in human prostate tumor material by two-dimensional gel electrophoresis and mass spectrometry. Cell Mol. Life Sci. 2001, 58, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Khaleque, M.A.; Jones, E.L.; Theriault, J.R.; Li, C.; Wong, W.H.; Stevenson, M.A.; Calderwood, S.K. Expression of heat shock proteins and heat shock protein messenger ribonucleic acid in human prostate carcinoma in vitro and in tumors in vivo. Cell Stress Chaperones 2005, 10, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P.; Wang, Q.X.; Li, H.Y.; Chen, R.F. Heat shock protein 70 chaperoned alpha-fetoprotein in human hepatocellular carcinoma cell line BEL-7402. World J. Gastroenterol. 2005, 11, 5561–5564. [Google Scholar] [CrossRef] [PubMed]
- Hellman, K.; Alaiya, A.A.; Schedvins, K.; Steinberg, W.; Hellstrom, A.C.; Auer, G. Protein expression patterns in primary carcinoma of the vagina. Br. J. Cancer 2004, 91, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Hunt, C.; Yaglom, J.A.; Gabai, V.L.; Sherman, M.Y. Heat shock protein Hsp72 plays an essential role in Her2-induced mammary tumorigenesis. Oncogene 2011, 30, 2836–2845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tetu, B.; Brisson, J.; Landry, J.; Huot, J. Prognostic significance of heat-shock protein-27 in node-positive breast carcinoma: An immunohistochemical study. Breast Cancer Res. Treat. 1995, 36, 93–97. [Google Scholar] [CrossRef]
- Kang, S.H.; Kang, K.W.; Kim, K.H.; Kwon, B.; Kim, S.K.; Lee, H.Y.; Kong, S.Y.; Lee, E.S.; Jang, S.G.; Yoo, B.C. Upregulated HSP27 in human breast cancer cells reduces Herceptin susceptibility by increasing Her2 protein stability. BMC Cancer 2008, 8, 286. [Google Scholar] [CrossRef]
- Geisler, J.P.; Tammela, J.E.J.E.; Manahan, K.J.; Geisler, H.E.; Miller, G.A.G.A.; Zhou, Z.; Wiemann, M.C. HSP27 in patients with ovarian carcinoma: Still an independent prognostic indicator at 60 months follow-up. Eur. J. Gynaecol. Oncol. 2004, 25, 165–168. [Google Scholar]
- Giaginis, C.; Daskalopoulou, S.S.; Vgenopoulou, S.; Sfiniadakis, I.; Kouraklis, G.; Theocharis, S.E. Heat shock protein-27, -60 and -90 expression in gastric cancer: Association with clinicopathological variables and patient survival. BMC Gastroenterol. 2009, 9, 14. [Google Scholar]
- Foster, C.S.; Dodson, A.R.A.R.; Ambroisine, L.; Fisher, G.; Moller, H.; Clark, J.J.; Attard, G.; De-Bono, J.; Scardino, P.; Reuter, V.E.V.E.; et al. Hsp-27 expression at diagnosis predicts poor clinical outcome in prostate cancer independent of ETS-gene rearrangement. Br. J. Cancer 2009, 101, 1137–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Z.; Zhi, J.; Peng, X.; Zhong, X.; Xu, A. Clinical significance of HSP27 expression in colorectal cancer. Mol. Med. Rep. 2010, 3, 953–958. [Google Scholar]
- Tweedle, E.M.; Khattak, I.; Ang, C.W.; Nedjadi, T.; Jenkins, R.; Park, B.K.; Kalirai, H.; Dodson, A.; Azadeh, B.; Terlizzo, M.; et al. Low molecular weight heat shock protein HSP27 is a prognostic indicator in rectal cancer but not colon cancer. Gut 2010, 59, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Ioachin, E. Immunohistochemical tumour markers in endometrial carcinoma. Eur. J. Gynaecol. Oncol. 2005, 26, 363–371. [Google Scholar] [PubMed]
- Storm, F.K.; Mahvi, D.M.; Gilchrist, K.W. Hsp-27 has no diagnostic or prognostic significance in prostate or bladder cancers. Urology 1993, 42, 379–382. [Google Scholar] [CrossRef]
- Ciocca, D.R.; Calderwood, S.K. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005, 10, 86–103. [Google Scholar] [CrossRef] [PubMed]
- Tavernier, E.; Duval, A.; Cornillon, J.; Flandrin, P.; Guyotat, D.; Campos, L. Prognostic value of CXCRCR4, adhesion molecules and heat shock proteins (HSP) in acute myelogenous leukemia. Blood (ASH Ann. Meet. Abstr.) 2007, 110, 2848. [Google Scholar]
- Alexiou, G.A.; Karamoutsios, A.; Lallas, G.; Ragos, V.; Goussia, A.; Kyritsis, A.P.; Voulgaris, S.; Vartholomatos, G. Expression of heat shock proteins in brain tumors. Turk. Neurosurg. 2014, 24, 745–749. [Google Scholar] [CrossRef]
| Factor | Effect of F | Contribution to Degenerative Eye Diseases |
|---|---|---|
| Enolase | ↓ | Loss of enolase induces cataractogenesis. τ-Crystallin, heat shock proteins, hypoxic stress proteins and c-Myc binding proteins possess enolase activity. These proteins are essential for lens function repair and protection. |
| Heat Shock Proteins | ||
| Hsp 40 | ↓ | Hsp 40 has been found to protect the lens from stress induced denaturation. |
| Hsp 27 | ↑ | Hsp27 expression associated with AMD and cataracts. |
| Hsp 70 | ↑ | Hsp70 expression associated with increased risk of cataracts and glaucoma |
| FoxO proteins | ↓ | FoxO proteins regulate antioxidant enzymes. Down regulation of FoxO1 and FoxO3a expression contributes to degenerative eye disorders such as cataract formation. |
| Na+, K+-ATPase | ↓ | Inhibition of Na+, K+-ATPase leads to enhanced ROS production and oxidative stress. Loss of Na+, K+-ATPase associated with cataractogenesis and age-dependent degeneration in photoreceptors, suggesting a link between loss of Na+, K+-ATPase and AMD. Loss of Na+, K+-ATPase linked to hypertension. Hypertension is a risk factor for cataracts, AMD and glaucoma. |
| PON1 | ↓ | PON1 is an antioxidant and reduces oxidative stress. Low PON1 activity associated with AMD and cataracts. |
| IL-6 | ↑ | IL-6 has been shown to be a key player in chronic low-grade systemic inflammation. Associated with cataracts, AMD and glaucoma. |
| Nrf2 | ↓ | Inhibition of dysregulation of Nrf2 pathway contributes to a state of chronic systemic inflammation with a diminished capacity to compensate for conditions of increased oxidative stress. Loss of Nrf2 is associated with AMD. |
| NF-kB | ↑ | NF-kB plays a critical role in the expression of inflammatory cytokines. Expression of NF-kB linked to AMD, cataracts and glaucoma. |
| BCL-2 | ↓ | Has anti-inflammatory properties, reduced expression associated with pathological states and degenerative eye diseases. |
| Antioxidants | ↓ | Impaired antioxidant activity leads to oxidative stress. Oxidative stress strongly associated with AMD, cataracts and glaucoma. |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waugh, D.T. The Contribution of Fluoride to the Pathogenesis of Eye Diseases: Molecular Mechanisms and Implications for Public Health. Int. J. Environ. Res. Public Health 2019, 16, 856. https://doi.org/10.3390/ijerph16050856
Waugh DT. The Contribution of Fluoride to the Pathogenesis of Eye Diseases: Molecular Mechanisms and Implications for Public Health. International Journal of Environmental Research and Public Health. 2019; 16(5):856. https://doi.org/10.3390/ijerph16050856
Chicago/Turabian StyleWaugh, Declan Timothy. 2019. "The Contribution of Fluoride to the Pathogenesis of Eye Diseases: Molecular Mechanisms and Implications for Public Health" International Journal of Environmental Research and Public Health 16, no. 5: 856. https://doi.org/10.3390/ijerph16050856
APA StyleWaugh, D. T. (2019). The Contribution of Fluoride to the Pathogenesis of Eye Diseases: Molecular Mechanisms and Implications for Public Health. International Journal of Environmental Research and Public Health, 16(5), 856. https://doi.org/10.3390/ijerph16050856