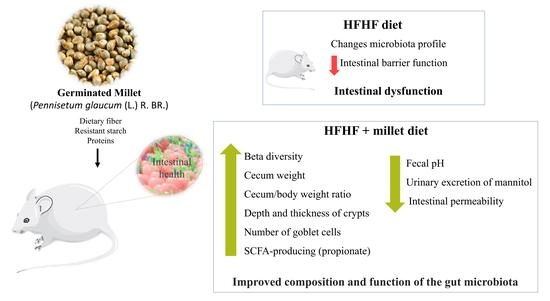
Germinated Millet (Pennisetum glaucum (L.) R. Br.) Flour Improved the Gut Function and Its Microbiota Composition in Rats Fed with High-Fat High-Fructose Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Grain Germination
2.2. Experimental Design, Animals, and Diets
2.3. Intestinal Permeability
2.4. Fecal pH
2.5. Short-Chain Fatty Acids (SCFA) Content
2.6. Colon Histomorphometry Analysis
2.7. DNA Extraction and Sequencing
2.8. Statistical Analysis
3. Results
3.1. Effect of Germinated Millet Flour on Food Intake, Body Weight, and Cecum Weight
3.2. Germinated Millet Flour Improves Intestinal Health
3.3. Effects of Germinated Millet Flour Consumption on the Diversity of the Microbial Community
3.4. Microbial Correlation with Markers of Metabolic Alterations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ter Horst, K.W.; Serlie, M.J. Fructose Consumption, Lipogenesis, and Non-Alcoholic Fatty Liver Disease. Nutrients 2017, 9, 981. [Google Scholar] [CrossRef] [Green Version]
- Balakumar, M.; Raji, L.; Prabhu, D.; Sathishkumar, C.; Prabu, P.; Mohan, V.; Balasubramanyam, M. High-Fructose Diet Is as Detrimental as High-Fat Diet in the Induction of Insulin Resistance and Diabetes Mediated by Hepatic/Pancreatic Endoplasmic Reticulum (ER) Stress. Mol. Cell. Biochem. 2016, 423, 93–104. [Google Scholar] [CrossRef]
- Lozano, I.; Van Der Werf, R.; Bietiger, W.; Seyfritz, E.; Peronet, C.; Pinget, M.; Jeandidier, N.; Maillard, E.; Marchioni, E.; Sigrist, S.; et al. High-Fructose and High-Fat Diet-Induced Disorders in Rats: Impact on Diabetes Risk, Hepatic and Vascular Complications. Nutr. Metab. 2016, 13, 15. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.G.; Hur, K.Y.; Lee, M.S. Alterations in Gut Microbiota and Immunity by Dietary Fat. Yonsei Med. J. 2017, 58, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Amar, J.; Chabo, C.; Waget, A.; Klopp, P.; Vachoux, C.; Bermúdez-Humarán, L.G.; Smirnova, N.; Bergé, M.; Sulpice, T.; Lahtinen, S.; et al. Intestinal Mucosal Adherence and Translocation of Commensal Bacteria at the Early Onset of Type 2 Diabetes: Molecular Mechanisms and Probiotic Treatment. EMBO Mol. Med. 2011, 3, 559–572. [Google Scholar] [CrossRef]
- Scheithauer, T.P.M.; Dallinga-Thie, G.M.; de Vos, W.M.; Nieuwdorp, M.; van Raalte, D.H. Causality of Small and Large Intestinal Microbiota in Weight Regulation and Insulin Resistance. Mol. Metab. 2016, 5, 759–770. [Google Scholar] [CrossRef]
- Konikoff, T.; Gophna, U. Oscillospira: A Central, Enigmatic Component of the Human Gut Microbiota. Trends Microbiol. 2016, 24, 523–524. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Pan, M.; Pan, S.; Li, W.; Zhong, Y.; Hu, J.; Nie, S. Effects of Insoluble and Soluble Fibers Isolated from Barley on Blood Glucose, Serum Lipids, Liver Function and Caecal Short-Chain Fatty Acids in Type 2 Diabetic and Normal Rats. Food Chem. Toxicol. 2020, 135, 110937. [Google Scholar] [CrossRef]
- Adam, C.L.; Williams, P.A.; Garden, K.E.; Thomson, L.M.; Ross, A.W. Dose-Dependent Effects of a Soluble Dietary Fibre (Pectin) on Food Intake, Adiposity, Gut Hypertrophy and Gut Satiety Hormone Secretion in Rats. PLoS ONE 2015, 10, e0115438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Song, P.; Fan, P.; He, T.; Jacobs, D.; Levesque, C.L.; Johnston, L.J.; Ji, L.; Ma, N.; Chen, Y.; et al. Moderate Dietary Protein Restriction Optimized Gut Microbiota and Mucosal Barrier in Growing Pig Model. Front. Cell. Infect. Microbiol. 2018, 8, 246. [Google Scholar] [CrossRef]
- Dias-Martins, A.M.; Pessanha, K.L.F.; Pacheco, S.; Rodrigues, J.A.S.; Carvalho, C.W.P. Potential Use of Pearl Millet (Pennisetum glaucum (L.) R. Br.) in Brazil: Food Security, Processing, Health Benefits and Nutritional Products. Food Res. Int. 2018, 109, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Gujral, H.S. Influence of Nutritional and Antinutritional Components on Dough Rheology and in Vitro Protein & Starch Digestibility of Minor Millets. Food Chem. 2019, 299, 125115. [Google Scholar] [CrossRef]
- Taylor, J.R. Millet Pearl: Overview. In Encyclopedia of Food Grains: Second Edition; Colin Wrigley: Brisbane, Australia, 2015; ISBN 9780123947864. [Google Scholar]
- Owheruo, J.O.; Ifesan, B.O.T.; Kolawole, A.O. Physicochemical Properties of Malted Finger Millet (Eleusine coracana) and Pearl Millet (Pennisetum glaucum). Food Sci. Nutr. 2019, 7, 476–482. [Google Scholar] [CrossRef] [Green Version]
- Sarita. Ekta singh Potential of Millets: Nutrients Composition and Health Benefits. J. Sci. Innov. Res. JSIR 2016, 5, 46–50. [Google Scholar] [CrossRef]
- Theodoro, J.M.V.; Martinez, O.D.M.; Grancieri, M.; Toledo, R.C.L.; Dias Martins, A.M.; Dias, D.M.; Carvalho, C.W.P.; Martino, H.S.D. Germinated Millet Flour (Pennisetum glaucum (L.) R. Br.) Reduces Inflammation, Oxidative Stress, and Liver Steatosis in Rats Fed with High-Fat High-Fructose Diet. J. Cereal Sci. 2021, 99, 103207. [Google Scholar] [CrossRef]
- Theodoro, J.M.V.; Martinez, O.D.M.; Grancieri, M.; Toledo, R.C.L.; Binoti, M.L.; Martins, A.M.D.; Carvalho, C.W.P.; Lisboa, P.C.; Martino, H.S.D. Germinated Millet Flour: (Pennisetum glaucum (L.) R. BR.) Improves Adipogenesis and Glucose Metabolism and Maintains Thyroid Function in Vivo. Food Funct. 2021, 12, 6083–6090. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 20th ed.; AOAC: Gaithersbg, MD, USA, 2016; p. 3172. [Google Scholar]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [Green Version]
- Marineli, R.d.S.; Moura, C.S.; Moraes, É.A.; Lenquiste, S.A.; Lollo, P.C.B.; Morato, P.N.; Amaya-Farfan, J.; Maróstica, M.R. Chia (Salvia hispanica L.) Enhances HSP, PGC-1α Expressions and Improves Glucose Tolerance in Diet-Induced Obese Rats. Nutrition 2015, 31, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Zhang, R.; Wang, X.; He, P.; Tan, L.; Ma, X. Dietary Grape-Seed Procyanidins Decreased Postweaning Diarrhea by Modulating Intestinal Permeability and Suppressing Oxidative Stress in Rats. J. Agric. Food Chem. 2011, 59, 6227–6232. [Google Scholar] [CrossRef]
- de Sá, L.R.V.; de Oliveira, M.A.L.; Cammarota, M.C.; Matos, A.; Ferreira-Leitão, V.S. Simultaneous Analysis of Carbohydrates and Volatile Fatty Acids by HPLC for Monitoring Fermentative Biohydrogen Production. Int. J. Hydrogen Energy 2011, 36, 15177–15186. [Google Scholar] [CrossRef]
- Garcia Vilela, E.; De Lourdes De Abreu Ferrari, M.; Oswaldo Da Gama Torres, H.; Guerra Pinto, A.; Carolina Carneiro Aguirre, A.; Paiva Martins, F.; Marcos Andrade Goulart, E.; Sales Da Cunha, A. Influence of Saccharomyces Boulardii on the Intestinal Permeability of Patients with Crohn’s Disease in Remission. Scand. J. Gastroenterol. 2008, 43, 842–848. [Google Scholar] [CrossRef]
- Grancieri, M.; Costa, N.M.B.; das Vaz Tostes, M.G.; de Oliveira, D.S.; de Nunes, L.C.; de Marcon, L.N.; Veridiano, T.A.; Viana, M.L. Yacon Flour (Smallanthus sonchifolius) Attenuates Intestinal Morbidity in Rats with Colon Cancer. J. Funct. Foods 2017, 37, 666–675. [Google Scholar] [CrossRef]
- Smiricky-Tjardes, M.R.; Grieshop, C.M.; Flickinger, E.A.; Bauer, L.L.; Fahey, G.C. Dietary Galactooligosaccharides Affect Ileal and Total-Tract Nutrient Digestibility, Ileal and Fecal Bacterial Concentrations, and Ileal Fermentative Characteristics of Growing Pigs1. J. Anim. Sci. 2003, 81, 2535–2545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, M.J.C.; da Silva, J.S.; Alves, N.E.G.; de Assis, A.; de Mejía, E.G.; Mantovani, H.C.; Martino, H.S.D. Cooked Common Bean Flour, but Not Its Protein Hydrolysate, Has the Potential to Improve Gut Microbiota Composition and Function in BALB/c Mice Fed a High-Fat Diet Added with 6-Propyl-2-Thiouracil. J. Nutr. Biochem. 2022, 106, 109022. [Google Scholar] [CrossRef]
- Stevenson, D.M.; Weimer, P.J. Dominance of Prevotella and Low Abundance of Classical Ruminal Bacterial Species in the Bovine Rumen Revealed by Relative Quantification Real-Time PCR. Appl. Microbiol. Biotechnol. 2007, 75, 165–174. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global Patterns of 16S RRNA Diversity at a Depth of Millions of Sequences per Sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-High-Throughput Microbial Community Analysis on the Illumina HiSeq and MiSeq Platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [Green Version]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozupone, C.; Knight, R. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef] [Green Version]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Lam, Y.Y.; Ha, C.W.Y.; Campbell, C.R.; Mitchell, A.J.; Dinudom, A.; Oscarsson, J.; Cook, D.I.; Hunt, N.H.; Caterson, I.D.; Holmes, A.J.; et al. Increased Gut Permeability and Microbiota Change Associate with Mesenteric Fat Inflammation and Metabolic Dysfunction in Diet-Induced Obese Mice. PLoS ONE 2012, 7, e34233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Wu, T.; Li, N.; Wang, X.; Chen, G.; Lyu, X. Bilberry Anthocyanin Extract Promotes Intestinal Barrier Function and Inhibits Digestive Enzyme Activity by Regulating the Gut Microbiota in Aging Rats. Food Funct. 2019, 10, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.J.C.; da Silva, J.S.; Assis, A.; de Mejia, E.G.; Mantovani, H.C.; Martino, H.S.D. Common Bean (Phaseolus vulgaris L.) Flour Can Improve the Gut Microbiota Composition and Function in Mice Fed a High-Fat Diet. Curr. Dev. Nutr. 2021, 5, 1159. [Google Scholar] [CrossRef]
- Moraes, É.A.; Natal, D.I.G.; Queiroz, V.A.V.; Schaffert, R.E.; Cecon, P.R.; de Paula, S.O.; dos Benjamim, L.A.; Ribeiro, S.M.R.; Martino, H.S.D. Sorghum Genotype May Reduce Low-Grade Inflammatory Response and Oxidative Stress and Maintains Jejunum Morphology of Rats Fed a Hyperlipidic Diet. Food Res. Int. 2012, 49, 553–559. [Google Scholar] [CrossRef] [Green Version]
- Hwang, D.; Koh, J.H.; Lee, J.; Kim, Y.; Lim, Y.H. Cherry Tomato Supplementation Increases the Area of the Intestinal Mucosa and the Number of Muscle Layers in Rats. Food Res. Int. 2014, 64, 298–304. [Google Scholar] [CrossRef]
- da Silva, B.P.; Toledo, R.C.L.; Grancieri, M.; de Moreira, M.E.C.; Medina, N.R.; Silva, R.R.; Costa, N.M.B.; Martino, H.S.D. Effects of Chia (Salvia hispanica L.) on Calcium Bioavailability and Inflammation in Wistar Rats. Food Res. Int. 2019, 116, 592–599. [Google Scholar] [CrossRef]
- Medina Martinez, O.D.; Vieira Theodoro, J.M.; Grancieri, M.; Lopes Toledo, R.C.; Vieira Queiroz, V.A.; Ribeiro de Barros, F.A.; Duarte Martino, H.S. Dry Heated Whole Sorghum Flour (BRS 305) with High Tannin and Resistant Starch Improves Glucose Metabolism, Modulates Adiposity, and Reduces Liver Steatosis and Lipogenesis in Wistar Rats Fed with a High-Fat High-Fructose Diet. J. Cereal Sci. 2021, 99, 103201. [Google Scholar] [CrossRef]
- Grancieri, M.; Verediano, T.A.; Sant’Ana, C.T.; de Assis, A.; Toledo, R.L.; de Mejia, E.G.; Martino, H.S.D. Digested Protein from Chia Seed (Salvia hispanica L) Prevents Obesity and Associated Inflammation of Adipose Tissue in Mice Fed a High-Fat Diet. PharmaNutrition 2022, 21, 100298. [Google Scholar] [CrossRef]
- Cardoso, R.R.; Moreira, L.D.P.D.; De Campos Costa, M.A.; Toledo, R.C.L.; Grancieri, M.; Do Nascimento, T.P.; Ferreira, M.S.L.; Da Matta, S.L.P.; Eller, M.R.; Duarte Martino, H.S.; et al. Kombuchas from Green and Black Teas Reduce Oxidative Stress, Liver Steatosis and Inflammation, and Improve Glucose Metabolism in Wistar Rats Fed a High-Fat High-Fructose Diet. Food Funct. 2021, 12, 10813–10827. [Google Scholar] [CrossRef]
- Stewart, G.S.; Smith, C.P. Urea Nitrogen Salvage Mechanisms and Their Relevance to Ruminants, Non-Ruminants and Man. Nutr. Res. Rev. 2005, 18, 49–62. [Google Scholar] [CrossRef]
- Hong, M.K.; Liu, H.H.; Chen, G.H.; Zhu, J.Q.; Zheng, S.Y.; Zhao, D.; Diao, J.; Jia, H.; Zhang, D.D.; Chen, S.X.; et al. Oridonin Alters Hepatic Urea Cycle via Gut Microbiota and Protects against Acetaminophen-Induced Liver Injury. Oxid. Med. Cell. Longev. 2021, 2021, 3259238. [Google Scholar] [CrossRef] [PubMed]
- Hithamani, G.; Srinivasan, K. Effect of Domestic Processing on the Polyphenol Content and Bioaccessibility in Finger Millet (Eleusine coracana) and Pearl Millet (Pennisetum Glaucum). Food Chem. 2014, 164, 55–62. [Google Scholar] [CrossRef] [PubMed]







| Variables | AIN-93M | HFHF | HFHF + Millet |
|---|---|---|---|
| Fecal pH | 9.18 ± 0.26 a | 9.28 ± 0.16 a | 8.63 ± 0.38 b |
| Propionic short-chain fatty acid (mM) | 4.58 ± 1.13 b | 4.36 ± 1.55 b | 9.96 ± 3.64 a |
| Acetic short-chain fatty acid (mM) | 21.36 ± 5.11 a | 13.71 ± 2.90 b | 13.81 ± 3.05 b |
| Butyric short-chain fatty acid (mM) | 2.51 ± 0.77 a | 3.60 ± 1.01 a | 3.19 ± 0.82 a |
| Number of goblet cells | 24.64 ± 3.93 b | 23.88 ± 3.91 b | 30.57 ± 2.10 a |
| Crypt’s depth (µM) | 127.2 ± 12.06 b | 134.3 ± 12.21 b | 168.7 ± 22.34 a |
| Crypt’s thickness (µM) | 18.27 ± 1.54 ab | 17.16 ± 1.79 b | 21.12 ± 2.22 a |
| Longitudinal mucus layer width (µM) | 86.51 ± 22.88 a | 74.05 ± 27.48 a | 81.93 ± 5.81 a |
| Circular mucus layer width (µM) | 28.50 ± 7.72 a | 25.99 ± 8.36 a | 32.41 ± 8.69 a |
| Mannitol urinary excretion (%) | 2.82 ± 0.85 b | 9.37 ± 6.70 a | 4.67 ± 2.09 b |
| Lactulose urinary excretion (%) | 5.44 ± 1.37 a | 9.07 ± 2.99 a | 10.03 ± 4.91 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theodoro, J.M.V.; Grancieri, M.; Gomes, M.J.C.; Toledo, R.C.L.; de São José, V.P.B.; Mantovani, H.C.; Carvalho, C.W.P.; da Silva, B.P.; Martino, H.S.D. Germinated Millet (Pennisetum glaucum (L.) R. Br.) Flour Improved the Gut Function and Its Microbiota Composition in Rats Fed with High-Fat High-Fructose Diet. Int. J. Environ. Res. Public Health 2022, 19, 15217. https://doi.org/10.3390/ijerph192215217
Theodoro JMV, Grancieri M, Gomes MJC, Toledo RCL, de São José VPB, Mantovani HC, Carvalho CWP, da Silva BP, Martino HSD. Germinated Millet (Pennisetum glaucum (L.) R. Br.) Flour Improved the Gut Function and Its Microbiota Composition in Rats Fed with High-Fat High-Fructose Diet. International Journal of Environmental Research and Public Health. 2022; 19(22):15217. https://doi.org/10.3390/ijerph192215217
Chicago/Turabian StyleTheodoro, Jaqueline Maciel Vieira, Mariana Grancieri, Mariana Juste Contin Gomes, Renata Celi Lopes Toledo, Vinícius Parzanini Brilhante de São José, Hilário Cuquetto Mantovani, Carlos Wanderlei Piler Carvalho, Bárbara Pereira da Silva, and Hércia Stampini Duarte Martino. 2022. "Germinated Millet (Pennisetum glaucum (L.) R. Br.) Flour Improved the Gut Function and Its Microbiota Composition in Rats Fed with High-Fat High-Fructose Diet" International Journal of Environmental Research and Public Health 19, no. 22: 15217. https://doi.org/10.3390/ijerph192215217
APA StyleTheodoro, J. M. V., Grancieri, M., Gomes, M. J. C., Toledo, R. C. L., de São José, V. P. B., Mantovani, H. C., Carvalho, C. W. P., da Silva, B. P., & Martino, H. S. D. (2022). Germinated Millet (Pennisetum glaucum (L.) R. Br.) Flour Improved the Gut Function and Its Microbiota Composition in Rats Fed with High-Fat High-Fructose Diet. International Journal of Environmental Research and Public Health, 19(22), 15217. https://doi.org/10.3390/ijerph192215217