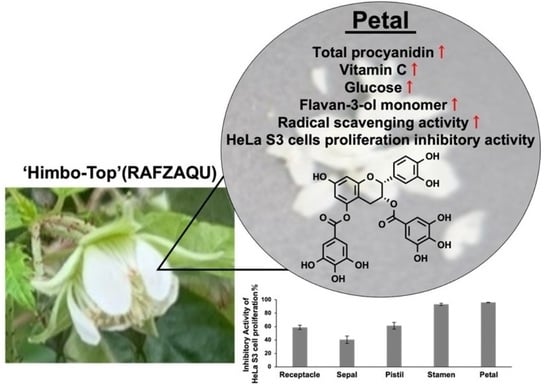
Potential of Raspberry Flower Petals as a Rich Source of Bioactive Flavan-3-ol Derivatives Revealed by Polyphenolic Profiling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Cultivation of Raspberries and Collection of Flower Samples
2.3. Preparation of Polyphenol Crude Extract
2.4. Determination of Total Polyphenol Content
2.5. Determination of Total Proanthocyanidin Content
2.6. Quantitation of Vitamin C Content
2.7. Quantitation of Total Glucose Content
2.8. HPLC and LC-MS Analyses
2.9. DPPH Radical Scavenging Activity
2.10. ABTS Radical Scavenging Activity
2.11. Lipid Peroxidation Inhibitory Activity Using TBA Method
2.12. Inhibitory Activity against Cervical Cancer (HeLa S3) Cells
2.13. Statistical Analysis
3. Results
3.1. Separation of Flowers into Parts and Extraction
3.2. Determination of Compounds Contained in Extracts
3.3. HPLC Analysis of Extracts and Identification of Compound Structures
3.4. Quantitation of Polyphenolics in Raspberry Flowers
3.5. Biological Activities of Raspberry Flower Extracts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt |
| BHT | butylated hydroxytoluene |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| EGCG | (−)-Epigallocatechin-3-O-gallate |
| HPLC | High-performance liquid chromatography |
| PE | petal |
| PI | pistil |
| RE | receptacle |
| SE | sepal |
| SDS | sodium Dodecyl Sulfate |
| ST | stamen |
| TEA | triethanolamine |
| TBA | thiobarbituric acid |
| VE | Vitamin E |
References
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, V.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Magnani, C.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic acid: A review of its potential use in medications and cosmetics. Anal. Methods 2014, 6, 3203–3210. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzai, M.; Ahmad, F.; Babazadeh, D.; Fang, X.F.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Zhang, L.X.; Li, C.X.; Kakar, M.U.; Khan, M.S.; Wu, P.F.; Amir, R.M.; Dai, D.F.; Naveed, M.; Li, Q.Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 12164. [Google Scholar] [CrossRef] [PubMed]
- Vattem, D.A.; Shetty, K. Biological functionality of ellagic acid: A review. J. Food Biochem. 2005, 29, 234–266. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Rasoulia, H.; Farzaeib, M.H.; Khodarahm, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, S1700–S1741. [Google Scholar] [CrossRef]
- Negri, A.; Valeria Naponelli, V.; Rizzi, F.; Bettuzzi, S. Molecular targets of epigallocatechin—Gallate (EGCG): A special focus on signal transduction and cancer. Nutrients 2018, 10, 1936. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Sivamaruthi, B.S.; Chaiyasut, C.; Tencomnao, T. A Review of the role of green tea (Camellia sinensis) in antiphotoaging, stress resistance, neuroprotection, and autophagy. Nutrients 2019, 11, 474. [Google Scholar] [CrossRef]
- Ángeles, M.; Ramos, M.S. Health beneficial effects of cocoa phenolic compounds: A mini-review. Curr. Opin. Food Sci. 2017, 14, 20–25. [Google Scholar]
- German, J.B.; Rosemary, L.; Walzem, R.L. The health benefits of wine. Annu. Rev. Nutr. 2000, 20, 561–593. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Haq, I.U.; Patel, S.; Pan, X.; Naz, S.; Silva, A.S.; Saeed, F.; Suleria, H.A.R. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Mizushina, Y.; Tanaka, A.; Nakajima, N. Versatile synthesis of epicatechin series procyanidin oligomers, and their antioxidant and DNA polymerase inhibitory activity. Tetrahedron 2009, 65, 7422–7428. [Google Scholar] [CrossRef]
- Higashino, Y.; Okamoto, T.; Mori, K.; Kawasaki, T.; Hamada, M.; Nakajima, N.; Saito, A. Regioselective synthesis of procyanidin B6, a 4-6-condensed (+)-catechin dimer, by intramolecular condensation. Molecules 2018, 23, 205. [Google Scholar] [CrossRef]
- Hamada, Y.; Takano, S.; Ayano, Y.; Tokunaga, M.; Koashi, T.; Okamoto, S.; Doi, S.; Ishida, M.; Kawasaki, T.; Hamada, M.; et al. Structure–activity relationship of oligomeric flavan-3-ols: Importance of the upper-unit B-ring hydroxyl groups in the dimeric structure for strong activities. Molecules 2015, 20, 18870–18885. [Google Scholar] [CrossRef]
- Mori, K.; Ayano, Y.; Hamada, Y.; Hojima, T.; Tanaka, R.; Higashino, Y.; Izuno, M.; Okamoto, T.; Kawasaki, T.; Hamada, M.; et al. Role of 2,3-cis structure of (−)-epicatechin-3,5-O-digallate in inhibition of HeLa S3 cell proliferation. Nat. Prod. Chem. Res. 2015, 3, 172. [Google Scholar]
- Bonyadi, N.; Dolatkhah, N.; Salekzamani, Y.; Hashemian, M. Effect of berry-based supplements and foods on cognitive function: A systematic review. Sci. Rep. 2022, 12, 3239. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Y.; Qin, Y.; Wang, L.; Wu, Z. HPLC-ESI-qTOF-MS/MS characterization, antioxidant activities and inhibitory ability of digestive enzymes with molecular docking analysis of various parts of raspberry (Rubus ideaus L.). Antioxidants 2019, 8, 274. [Google Scholar] [CrossRef]
- Kobori, R.; Hashimoto, S.; Koshimizu, H.; Yakami, S.; Hirai, M.; Noro, K.; Kawasaki, T.; Saito, A. Flavan-3-ols content in red raspberry leaves increases under blue LED-light irradiation. Metabolites 2019, 9, 56. [Google Scholar] [CrossRef] [PubMed]
- Kobori, R.; Yakami, S.; Kawasaki, T.; Saito, A. Changes in the polyphenol content of red raspberry fruits during ripening. Horticulturae 2021, 7, 569. [Google Scholar] [CrossRef]
- Folin, O.; Denis, W.A. Colorimetric method for the determination of phenols (and derivatives) in urine. J. Biol. Chem. 1915, 22, 305–308. [Google Scholar] [CrossRef]
- Tiitto, R.J. Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Geremu, M.; Tola, Y.B.; Sualeh, A. Extraction and determination of total polyphenols and antioxidant capacity of red coffee (Coffea arabica L.) pulp of wet processing plants. Chem. Biol. Technol. Agric. 2016, 3, 25. [Google Scholar] [CrossRef]
- Harbertson, J.F.; Kennedy, J.A.; Adams, D.O. Tannin in skin and seeds of cabernet sauvignon, syrah, and pinoy noir berries during ripening. Am. J. Enol. Vitic. 2002, 52, 54–59. [Google Scholar] [CrossRef]
- Mella, A.C.; Neira, A.P.; Bastias, J.N.; Campos, C.J.; Solis, R.L.; Canals, J.M. Comparison of analytical methods for measuring proanthocyanidins in wine and their relationship with perceived astringency. Int. J. Food Sci. Technol. 2013, 48, 2588–2594. [Google Scholar] [CrossRef]
- Kishida, E.; Nishimoto, Y.; Kojo, S. Specific determination of ascorbic acid with chemical derivatization and high-performance liquid chromatography. Anal. Chem. 1992, 64, 1505–1507. [Google Scholar] [CrossRef]
- Nanjo, F.; Goto, K.; Seto, R.; Suzuki, M.; Sakai, M.; Hara, Y. Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Radic. Biol. Med. 1996, 21, 895–902. [Google Scholar] [CrossRef]
- Pellegrini, R.R.N.; Proteggente, A.; Pannala, A.; Yang, M.; Evans, C.R. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 9–10. [Google Scholar]
- Saito, A.; Doi, Y.; Tanaka, A.; Matsuura, N.; Ubukata, M.; Nakajima, N. Systematic synthesis of four epicatechin series procyanidin trimers and their inhibitory activity on the Maillard reaction and antioxidant activity. Bioorg. Med. Chem. 2004, 12, 4783–4790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Dong, Y.; Chen, X.; Tan, C.S.H.; Li, M.; Miao, K.; Lu, J.H. Toosendanin, a late-stage autophagy inhibitor, sensitizes triple-negative breast cancer to irinotecan chemotherapy. Clin. Med. 2022, 17, 55. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Prabhu, V.; Mohanty, V.; Krishnaraj, U.; Abdulla, R. Unveiling the arcanum of formalin-fixed paraffin-embedded archival tissue blocks: A valuable resource for genomic DNA extraction. J. Oral Maxillofac. Pathol. 2022, 26, 289. [Google Scholar] [PubMed]
- Kušnierová, P.; Zeman, D.; Hradilic, P.; Čábal, M.; Zapletalová, O. Neurofilament levels in patients with neurological diseases: A comparison of neurofilament light and heavy chain levels. J. Clin. Lab. Anal. 2019, 33, e22948. [Google Scholar] [CrossRef]
- Yang, J.; Cui, J.; Chen, J.; Yao, J.; Hao, Y.; Fan, Y.; Liu, Y. Evaluation of physicochemical properties in three raspberries (Rubus ideaus) at five ripening stages in northern China. Sci. Hortic. 2020, 263, 109146. [Google Scholar] [CrossRef]
- Li, X.; Sun, J.; Chen, Z.; Jiang, J.; Jackson, A. Metabolite profile and genes/proteins expression in b-citraturin biosynthesis during fruit ripening in Chinese raspberry (Rubus chingi Hu). Plant Physiol. Biochem. 2021, 163, 76–86. [Google Scholar] [CrossRef]
- Mojzer, E.B.; Hrnčič, M.K.; Škerget, M.; Knez, Z.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Wan, H.; Yu, C.; Han, Y.; Guo, X.; Ahmed, S.; Tang, A.; Wang, J.; Cheng, T.; Pen, H.; Shang, Q. Flavonols and carotenoids in yellow petals of rose cultivar (Rosa ‘Sun City’): A possible rich source of bioactive compounds. J. Agric. Food Chem. 2018, 66, 4171–4181. [Google Scholar] [CrossRef]
- Renai, L.; Scordo, C.V.A.; Chiuminatto, U.; Ulaszewska, M.; Giordani, E.; Petrucci, W.A.; Tozzi, F.; Nin, S.; Bubba, M.D. Liquid chromatographic quadrupole time-of-flight mass spectrometric untargeted profiling of (poly)phenolic compounds in Rubus idaeus L. and ubus occidentalis L. Fruits and their comparative evaluation. Antioxidants 2021, 10, 704. [Google Scholar] [CrossRef]
- Saijo, R.; Takeda, Y. HPLC analysis of catechins in various kinds of green teas produced in Japan and abroad. Nippo Shokuhin Kagaku Kogaku Kaishi 1999, 46, 138–147. [Google Scholar] [CrossRef]
- Xie, L.; Yan, Z.; Li, M.; Tian, Y.; Kilaru, A.; Niu, L.; Zhang, Y. Identification of phytochemical markers for quality evaluation of tree peony stamen using comprehensive HPLC-based analysis. Ind. Crops Prod. 2020, 154, 112711. [Google Scholar] [CrossRef]
- Doge, R.; Nishino, Y.; Saito, A. Fluorinated derivatives of digalloyl-flavan-3-ol induce autophagic cell death by forming granular aggregates containing mitochondria. BioChem, 2023; in press. [Google Scholar]
- Li, Y.; Gan, G.P.; Zhang, H.Z.; Wu, H.Z.; Li, C.L.; Huang, Y.P.; Liu, Y.W.; Liua, J.W. A flavonoid glycoside isolated from Smilax china L. rhizome in vitro anticancer effects on human cancer cell lines. J. Ethnopharmacol. 2007, 113, 115–124. [Google Scholar] [CrossRef] [PubMed]






| RE | SE | PI | ST | PE | |
|---|---|---|---|---|---|
| Sample wet weight (g) | 1.03 | 0.60 | 0.0853 | 0.60 | 0.165 |
| Extract dry weight (g) | 0.482 | 0.394 | 0.0431 | 0.379 | 0.0245 |
| RE | SE | PI | ST | PE | |
|---|---|---|---|---|---|
| Dry weight of extract (g) 1 | 0.48 | 0.39 | 0.043 | 0.380 | 0.025 |
| (+)-Catechin (1) (mg) 2 | 0.13 | 0.13 | 0.22 | 0.14 | 0.83 |
| (−)-Epicatechin (2) (mg) 2 | 0.06 | 0.08 | 0.06 | 0.15 | 0.19 |
| Procyanidin B4 (3) (mg) 2 | 0.44 | 0.25 | 0.29 | 0.42 | 0.40 |
| Procyanidin C3 (4) (mg) 2 | 0.19 | 0.12 | 0.13 | 0.11 | 0.15 |
| (−)-Epicatechin-3,5-di-O-gallate (7) (mg) 2 | 0.02 | 0.02 | 0.02 | 0.03 | 0.08 |
| Kaempferol-7-O-glucoside (8) | nd 3 | nd | nd | nd | 0.03 |
| Naringenin-7-O-glucoside (9) | nd | nd | nd | nd | 0.03 |
| RE | SE | PI | ST | PE | |
|---|---|---|---|---|---|
| Total polyphenol 1 | ++ | ++ | ++ | +++ | ++ |
| Total proanthocyanidin 1 | ++ | - | + | +++ | ++ |
| Vitamin C 1 | ++ | ++ | +++ | +++ | +++ |
| Total glucose 1 | ++ | ++ | ++ | ++ | +++ |
| Flavan-3-ol mononers 1 | - | - | + | + | +++ |
| Procyanidins 1 | +++ | ++ | ++ | +++ | +++ |
| Radical scavenging activity 2 | +++ | ++ | ++ | +++ | +++ |
| Antioxidant activity 2 | +++ | +++ | +++ | +++ | +++ |
| HeLa S3 cell proliferation inhibitory activity 2 | ++ | ++ | ++ | +++ | +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobori, R.; Doge, R.; Takae, M.; Aoki, A.; Kawasaki, T.; Saito, A. Potential of Raspberry Flower Petals as a Rich Source of Bioactive Flavan-3-ol Derivatives Revealed by Polyphenolic Profiling. Nutraceuticals 2023, 3, 196-209. https://doi.org/10.3390/nutraceuticals3020015
Kobori R, Doge R, Takae M, Aoki A, Kawasaki T, Saito A. Potential of Raspberry Flower Petals as a Rich Source of Bioactive Flavan-3-ol Derivatives Revealed by Polyphenolic Profiling. Nutraceuticals. 2023; 3(2):196-209. https://doi.org/10.3390/nutraceuticals3020015
Chicago/Turabian StyleKobori, Ryo, Ryo Doge, Momoka Takae, Atoru Aoki, Takashi Kawasaki, and Akiko Saito. 2023. "Potential of Raspberry Flower Petals as a Rich Source of Bioactive Flavan-3-ol Derivatives Revealed by Polyphenolic Profiling" Nutraceuticals 3, no. 2: 196-209. https://doi.org/10.3390/nutraceuticals3020015
APA StyleKobori, R., Doge, R., Takae, M., Aoki, A., Kawasaki, T., & Saito, A. (2023). Potential of Raspberry Flower Petals as a Rich Source of Bioactive Flavan-3-ol Derivatives Revealed by Polyphenolic Profiling. Nutraceuticals, 3(2), 196-209. https://doi.org/10.3390/nutraceuticals3020015