1. Introduction
Cannabidiol (CBD) is the main non-psychoactive phytocannabinoid isolated from hemp, and in recent years its molecular targets have been intensely investigated to uncover its therapeutic potential. It has been demonstrated that CBD can interact with numerous receptors, including two cannabinoid receptors (CB1 and CB2) [
1] several non-cannabinoid receptors and ion channels. CBD acts as serotonin 1A receptor, regulating transient receptor potential vanilloid 1 (TRPV1), voltage-activated T-type calcium channels, adenosine and glycine receptors, µ- and δ-opioid receptors and voltage-dependent anion channel 1 [
2]. Consequently, many possible clinical applications are under investigation, including anti-anxiety properties, effects on appetite and sleep, pain perception, nausea and vomiting, inflammation, modulation of blood pressure and bone density and insulin sensitivity [
3,
4].
Up to now, the United States Food and Drug Administration (FDA), the European Medicines Agency (EMA) and other regulatory agencies have approved a drug based on CBD. It is Epidiolex, respectively approved by the FDA in 2018 [
5] and by the EMA in 2019 [
6]. Epidiolex consists of an oily dosage form (refined sesame oil plus anhydrous ethanol) prescription medicine containing 100 mg/mL CBD, indicating it to be an adjuvant treatment of seizures associated with Lennox-Gastaut syndrome, Dravet syndrome or tuberous sclerosis complex in patients of 2 years of age and older. As these syndromes are very rare diseases, Epidiolex was designated as an ‘orphan medicine’ by the EMA [
7].
Conversely, CBD is largely used as an ingredient in foods (candies, biscuits, herbal teas, flour and pasta) and food supplements worldwide, because of the numerous valuable health effects, principally its anti-inflammatory and antioxidant properties and being able to decrease oxidative situations by avoiding the creation of superoxide radicals [
8].
Accordingly, the urgent need to regulate the food use of CBD has recently attracted the attention of regulatory agencies and national and international organizations. The main advantage of CBD is the lack of psychoactive and psychotropic effects, unlike delta9-trans-tetrahydrocannabinol (THC). Around the world, specific regulations and guidelines with clear conditions on the use of CBD in food and food supplements are available. In some countries, safety assessments still need to be performed before products containing CBD ingredients can be legally marketed and in other countries CBD ingredients are still not permitted, or are highly restricted, for use in foods and beverages [
9]. Remarkably, the World Health Organization (WHO) issued a report in June 2018 concluding that there is no evidence of any public health-related problems associated with the use of pure CBD [
10]. This publication convinced the United Nations Commission on Narcotic Drugs to remove cannabis and cannabis resin from the list of narcotic substances, legitimizing (since December 2020) their use for medical and scientific purposes and reintegrating cannabinoids in the pharmacopoeia. For other uses, however, cannabis remains classified as a “risk” substance, along with other synthetic substances [
11].
In October 2020, the European Commission announced that natural CBD and its products should not be considered as a drug but might fall within the scope of the EU novel foods regulation (EU 2015/2283) since significant human consumption of CBD prior to 15 May 1997 was not demonstrated. A month later, the EU Court of Justice ruled that, “According to the current state of scientific knowledge, unlike THC, the CBD at issue does not seem to have any psychotropic effect or any harmful effect on human health” [
12]. Currently, CBD is included in the novel food catalogue [
13].
The UK Food Standards Agency (FSA) has classified CBD as novel food and proposed a human adult upper limit of 70 mg of CBD per day for foods [
14].
In the United States of America, CBD is now available in all 50 states to varying degrees, having access to the supplement in-store legally but it may be hard to find it in some of the stricter states that require medical cards. Several states, such as New York, Texas and more recently Virginia, are coming up with their own rules on the use of CBD or hemp extracts in food and dietary supplements, adding regulatory complexity to the market. The FDA has consistently declared that CBD cannot be lawfully marketed in food and dietary supplements because the cannabis-derived compound was first studied as a drug. At federal level, several notices have been presented at the US Congress to allow the use of hemp and hemp-derived CBD as an ingredient in food and dietary supplements [
15,
16]. Despite these initiatives at the federal level, the industry continues to request that the FDA carries out safety assessments on CBD and regulates it as an ingredient in food and dietary supplements. Currently, the food status of CBD is recognized in different countries of the world, but further clarification of regulatory challenges, as well as for the outcome of the ongoing safety assessments, is needed [
9]. According to this panorama, it is expected that an increasing number of food and food supplements based on CBD will gain access to markets worldwide. Indeed, some CBD biopharmaceutical issues should be overcome to market bio-accessible and safe products because CBD is a very unstable molecule—it is thermo-labile after heating or microwave irradiation and extraordinarily sensitive to oxidation. In addition, it is not stable in simulated physiological conditions (pH = 7.4, 37 °C) or in the presence of Bronstëd and Lewis acids [
17,
18]. These limitations, together with a pronounced pre-systemic metabolism [
19,
20], are significant, and the resulting oral bioavailability of CBD is very low (less than 6%), according to World Health Organization [
21]. Here, we have reported in a narrative review how CBD bioavailability can be improved after it is loaded into various colloidal delivery systems, including nanovectors. The paper evidenced how nanosized lipid formulations, including nanostructured lipid carriers, vesicles, SNEEDS, nanoemulsions and microemulsions, can be very successful in terms of high CBD solubility, encapsulation efficiency and stability and sustained CBD release [
22]. In addition, the simple architectures of the developed nanosystems together with the use of generally recognized safe excipients represent crucial steps for their use in humans [
23].
The purpose of this study was to investigate the loading properties of CBD in new nanostructured lipid carriers (NLCs), evaluating its bioaccessibility in gastric and intestinal simulated physiological medium. Hence, CBD is a highly lipophilic compound with a low solubility in the gastric and enteric media and high permeability through the epithelial membranes [
24]. According to the Biopharmaceutical Classification System developed by Amidon for the drugs [
25], CBD is a Class II molecule because it has a poor water solubility, but its permeability is high. Consequently, the absorption process is affected by the CBD dissolution in the gastrointestinal fluids, which is the rate-determining step and formulations should assist with the solubility increasing [
26].
The choice of NLCs as a nanodrug delivery system loading CBD was chosen because they can control the CBD release by the swelling process and/or dissolving process, and in addition, it is possible to maximize the CBD loading because they contain both solid and liquid lipids, which in turn produced less ordered lipid core and obtained an increased loading [
27].
2. Materials and Methods
2.1. Chemicals
Cannabidiol (CBD) was purchased from Galeno Srl (Prato, Italy). Compritol 888 ATO, lauroglycol 90 and labrafil 2125 were supplied by Gattefossè (Saint Priest, Germany). Tween 20, Poloxamer 188, calcium chloride, sodium chloride, sodium hydroxide, lipase from porcine pancreas, pancreatin from porcine pancreas, pepsin from porcine gastric mucosa, bile salts, phosphate-buffered saline, methanol, acetonitrile and formic acid of HPLC grade were from Sigma Aldrich (Milano, Italia). Ultrapure water was produced using a synergy UV Simplicity water purification system provided by Merck KGaA (Molsheim, France).
2.2. High Performance Liquid Chromatograph (HPLC) Analytical Method for CBD
The previously developed and validated HPLC method [
28] was adapted to the analysis of the settled NLCs using the same 1100 High Performance Liquid Chromatograph (HPLC), equipped with a Diode Array Detector (DAD) from Agilent Technologies Italia Spa (Rome, Italy). The same HPLC conditions, column and gradient were applied. Chromatograms were acquired at 210 nm. Calibration curve gave a coefficient of determination R
2 > 0.9999 using a standard solution of CBD in methanol (0.06 mg/mL) 10 and 100 folds diluted using different injection volumes (9, 7, 5, 3 µL). Limit of detection (LOD) and limit of quantification (LOQ) were 5.01 × 10
1 ng/mL and 5.01 × 10
2 ng/mL, respectively.
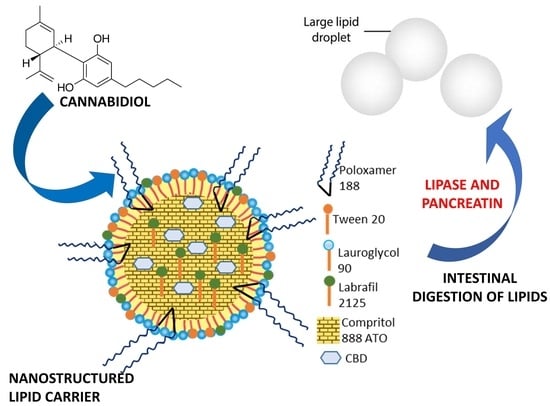
2.3. Preparation of NLCs and Optimization of CBD-Loaded NLCs
Different surfactants and lipids (Poloxamer 188, Tween 20, Lauroglycol 90 and Labraphyl M2125, Compritol 888 ATO) were screened to prepare the NLCs. Among the different components, Compritol 888 ATO and Poloxamer were solid, while the other excipients were viscous liquids. The quantity of total excipients in terms of percentage (
w/
v) was fixed at about 5% and the blend of solid and liquid excipients was fixed at a ratio of 7:3 [
27]. Fixed amounts of Poloxamer 188, Lauroglycol 90, Tween 20 and Labrafil M 2125 were set as 0.50% or 1.05%
w/
v, while Compritol 888 ATO was investigated in the range between 2.5 and 3.0%
w/
v. NLCs were prepared using the emulsification-ultrasonication method, as previously reported [
29].
Briefly, Poloxamer 188 was solubilized in ultrapure water, while Compritol 888 ATO was solubilized in the oily phase consisting of Tween 20 plus Lauroglycol 90 and Labraphyl M2125. The two mixtures were put in a water bath and melted at 85 ± 2 °C under magnetic stirring at 250 rpm for 20 min, allowing the components to melt or dissolve and giving them a homogeneous mix. A thermometric probe carefully controlled the bath temperatures. Successively, the two hot solutions were quickly mixed, adding the aqueous phase to the oil phase, and the mixture was emulsified using the T25 Ultra-Turrax (IKA; Khonigswinter, Germany) at 5000 rpm speed for 5 min. The resulting nanoemulsion was rapidly cooled down at a temperature below 4 °C using ice and formulation was optimized using the ultrasonic homogenizer Sonopuls HD 2200 (Bandelin Electronic GmbH; Berlin, Germany), which was coupled to the KE-76 probe. The ultrasonication process was carried out for 5 min using 3 mL of the sample, 45% amplitude and 2 pulser (output of 13 watts) at room temperature (25 ± 2 °C).
Among the investigated mixtures, the only one that produced an NLC was the following: 0.5% w/v of Poloxamer 188 and Labraphyl M2125, 1.0% w/v of Tween 20 and Lauroglycol 90 and 2.5% w/v of Compritol 888 ATO. CBD (10 mg/mL, 1% w/v) was added to the oil mixture before mixing the oily phase with water. CBD-NLC formulation was optimized by adjusting concentrations of the excipients to obtain maximum CBD encapsulation efficiency. The final concentrations of the different components in the NLC aqueous colloidal suspension were as follows: 1% w/v of CBD, 0.52% w/v of Poloxamer 188 and Labrafil M 2125, 2.36 % w/v of Compritol 888 ATO and 1.05% w/v of Lauroglycol 90 and Tween 20.
2.4. Lyophilization Process
About 3 mL of aqueous colloidal NLCs or CBD-NLCs was fast-frozen by using liquid nitrogen. Subsequently, the samples were freeze-dried for at least one day through the Leybold-Heraeus freeze-drier Lyovac GT (Leybold; Turin, Italy). The freeze-dried powder was used for calorimetric measurements.
2.5. Differential Scanning Calorimetric Analysis (DSC)
The thermograms of pure compounds and lyophilized NLC products were obtained using a Mettler TA4000 calorimeter equipped with a DSC25 cell (Mettler Toledo, Columbus, OH, USA). The samples, weighed with a Mettler M3 Microbalance (4–6 mg samples), were scanned in aluminium pans pierced with a perforated lid at 10 °C/min and heated under static air from 30 to 100 °C.
2.6. Dynamic and Electrophoretic Light Scattering Analysis
The samples were analyzed by the dynamic and electrophoretic light scattering (DLS and ELS) techniques using the Zetasizer Pro Red of Malvern Panalytical (Alfatest Srl, Milan, Italy) equipped with a He-Ne gas laser with a maximum output power of 10 mW and a beam wavelength of 632.8 nm, as well as with an avalanche photodiode (APD) detector. The measurements were performed at 25 °C, with a back scatter detection angle of 173°. The analyses allow for determining the samples’ average hydrodynamic diameter (size, nm), polydispersity index (PdI, dimensionless parameter) and ζ-potential (mV). Each measurement was carried out three times.
2.7. Determination of CBD Encapsulation Efficiency and Recovery
In order to characterize the CBD-NLC aqueous dispersions, the encapsulation efficiency (EE%) and the total recovery (R%) were calculated as reported above.
R% is defined as the percentage of total drug recovered after the preparation procedure in relation to the weighed drug (Equation (1), [
28]):
Before the analyses samples were diluted 1:100 with MeOH, ultrasonicated for 20 min at 40 °C and centrifuged for 10 min at 14,000 rpm. After the test, the supernatant was diluted 1:10, ultrasonicated for 10 min at 40 °C and centrifuged for 10 min at 14,000 rpm at room temperature.
EE is defined as the percentage of entrapped drug in relation to the weighed drug (Equation (2) [
28]):
Encapsulation efficiency (EE%) of CBD in the NLC was determined by the direct method of dialysis bag. Specifically, for the purification step, 2 mL of formulation were loaded into a Spectra/Por
® dialysis tubing of regenerated cellulose with molecular weight cut-off of 12–14 KDa (Repligen Europe B.V.; Breda, the Netherlands) and were dialyzed using 1 L of ultrapure water at room temperature (25 °C) in stirring conditions (100 rpm) for one hour, as previously reported [
30,
31]. Subsequently, the bag content was analysed as described above. In particular, the supernatants were analysed by HPLC-DAD.
2.8. Studies of CBD-NLC Stability in Gastric and Intestinal Simulated Fluids
The stability of CBD-NLC, in terms of CBD recovery (R%), size and PdI of nanoparticles, was evaluated in simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) by using 50 folds dilution [
29]. Briefly, the composition of SGF was 3.2 g of pepsin, 2.0 g of NaCl and 7 mL HCl dissolved in 1 L of ultrapure water (pH adjusted to 2.0 using 1 M NaOH). An aliquot of 100 μL of NLCs was diluted in SGF (dilution factor 1:50) and placed in the PST-60H-4 Plate Thermo-Shaker (Biosan; Riga; Latvia) at 37 °C under continuous stirring at 300 rpm for two hours. CBD-NLC was also diluted (1:50) in SIF (pH 7.0), comprising 0.4 mg/mL lipase, 0.7 mg/mL bile salts, 0.5 mg/mL pancreatin and 750 mM CaCl
2. This sample was incubated for six hours at 37 °C under magnetic stirring at 300 rpm. At the beginning of the incubation and every two hours the samples were collected and centrifuged for 1 min at 5000 rpm. The supernatant was diluted 1:2 in ultrapure water and analysed to measure size, PdI and R%. The total dilution of NLCs in SGF and SIF was 1:100 [
32,
33].
2.9. Statistical Analysis
The experiments were performed at least in triplicate. The results were expressed as the mean ± standard deviation (SD) or standard error (SE) of the mean. Descriptive statistical analysis and Bonferroni’s pairwise comparison of the means and graphs were made using Origin Pro 2022b [
34].
3. Results
3.1. Selection of Excipients
NLCs are made of solid lipids, liquid lipids, surfactants and/or cosurfactants and water. The first step of the present investigation was the selection of excipients to develop the NLCs for oral administration. Accordingly, GRAS (Generally Recognized As Safe) substances were selected. In particular, Compritol 888 ATO was chosen as a solid lipid because it is widely used in lipid-based colloidal drug delivery system, including solid lipid microparticles, solid lipid nanoparticles and nanostructured lipid carriers. Compritol 888 ATO consists of mono-, di- and tri-esters of behenic acid (C22), resulting in a melting point ranging from 65 to 77 °C. Lauroglycol 90 was also selected because it is a lipophilic nonionic water-insoluble surfactant (HLB 3) largely used in oral lipid-based formulations, principally to increase solubility and bioavailability for poorly soluble drugs. Lauroglycol 90 consists of propylene glycol mono- and di-esters of lauric acid (C12), mainly composed of monoesters and a small fraction of diesters. Additionally, Labrafil 2125 was included in the formula. It is a liquid nonionic water-dispersible surfactant (HLB 9), generally used for lipid-based formulations to solubilize and increase oral bioavailability of poorly water-soluble constituents. It consists of mono-, di- and triglycerides and PEG-6 (MW 300) mono- and diesters of linoleic (C18:2) acid.
Two further well-known hydrophilic surfactants were selected, namely Tween 20 (polyoxyethylene (20) sorbitan monolaurate, a liquid with HLB 16), Poloxamer 188 (a biocompatible block copolymer composed of repeating units of polyethylene oxide) and polypropylene oxide (solid excipient, HLB 29, melting point 52 °C).
3.2. NLC Development
NLCs were prepared by the emulsification-ultrasonication method, as reported in the experimental part. Among the tested mixtures, only the formulation containing CBD (1% w/v), Compritol 888 ATO (2.36% w/v), Lauroglycol 90 (1.05% w/v), Labrafil 2125 (0.52% w/v), Tween 20 (1.05% w/v), Poloxamer 188 (0.52% w/v) and ultrapure water (93.94% w/v) had the proper characteristics to be used for oral administration.
3.3. Physical and Chemical Characterization of NLCs and CBD-Loaded NLC
The samples were analyzed by dynamic and electrophoretic light scattering (DLS and ELS) to evaluate the average particle size, PdI and Z-potential of the NLCs and CBD-loaded NLCs, which resulted in them being suitable for oral administration [
35] (
Table 1). NLC dimensions after CBD loading increased slightly but without any significance, as reported by other studies [
29]. PdI of both formulations was less than 0.3, indicating worthy sample homogeneity. In addition, the PdI value reported for the CBD is superior with respect to reported for the unloaded NLCs. Moreover, the high ζ-potential (−39.21 ± 1.89 mV,
Table 1) of the CBD-loaded NLCs indicates a very high physical stability of the formulation preventing possible aggregation phenomena [
35,
36]. Recovery (R%) and encapsulation efficiency (EE%) of CBD in the loaded NLCs, determined by HPLC equipped with a DAD, are reported in
Table 1. The high value of R% (almost 100%) demonstrated that CBD is not degraded during nanoparticle preparation and the EE% value (ca. 93%) indicated the suitability of the formulation as drug delivery system of CBD.
3.4. Differential Scanning Calorimetric Analysis (DSC) of NLC
DSC was used to analyze the freeze-dried NLCs and evaluate the transition temperatures. The DSC curves of CBD-loaded NLCs, free NLCs, CBD, Poloxamer 188 and Compritol 888 ATO are reported in
Figure 1.
CBD (green line) exhibited an endothermic peak at 68 °C, Compritol 888 ATO (light blue line) at 74 °C and poloxamer 188 (blue line) at 58 °C, corresponding to their melting point peaks. Both the unloaded NLCs (red line) and the CBD-loaded NLCs (black line) display curves without the typical melting peaks of each ingredient, which confirmed that a new lipophilic system was formed, where each ingredient became an amorphous structure, with the exception of CBD, whose endothermic peak was still present in the NLCs.
3.5. Stability Studies of CBD-Loaded NLCs in Simulated Gastric and Intestinal Fluids
Generally, liposomes and emulsions fail to protect the encapsulated drug, whereas NLCs can protect the drug from degradation, as it is capable of loading the drug. CBD is reported to be very unstable in simulated physiological conditions (pH = 7.4, 37 °C) and in the presence of Bronstëd and Lewis acids [
22]. The aim of this study was to evidence the stability of the developed nanocarriers, the recovery of CBD in simulated gastric fluid (SGF) and simulated intestinal fluids (SIF) by measuring the size and PdI of the nanocarriers (
Figure 2), and R% of CBD after different incubation times (
Table 2). Size, PdI and R% were measured immediately after the beginning of the experiments, and then every two hours, until the end of the experiments, i.e., two and six hours for SGF and SIF, respectively.
Immediately after the dilution of the colloidal aqueous CBD-NLC with SGF, no modification of the values of size and PdI was found. In addition, CBD was not detected in the fluids. These data agreed with a high stability of CBD-NLC without release of CBD. By contrast, when the CBD-NLCs were put in SIF, the mean diameters of the nanovectors increased significantly, reaching a size of 575 nm, which became 1490 after four hours, and 2000 nm after six hours (
Figure 2). In addition, a significant modification of PdI value was found (
Figure 2). These results evidenced the enzymatic digestion of NLCs due to pepsin, pancreatin and lipase, and the consequent rearrangement of the NLCs in undefined aggregates or micelles, evidencing a physical stability of NLCs in SGF but not in SIF. This behaviour of NLCs in SIF was due to the special composition of the nanoparticles.
Indeed, Compritol 888 ATO and Lauroglycol 90, being mono-, di- and tri-esters of behenic acid (C22), and propylene glycol mono- and di-esters of lauric acid (C12), respectively, were prone to digestion. Labrafil 2125, Tween 20 and Poloxamer 188 (P188), because of their structural hindrance, could considerably affect the degradation rate, slowing it down but sometimes accelerating it, as in our experiments [
37]. The amount of CBD was monitored in both simulated media. No CBD was detected in the SGF, while it was detected in the SIF, evidencing an increasing amount during time. The CBD R% reached 100% after six hours (
Table 2). These results agree with the physical stability study of CBD-NLC in SGF. By contrast, in intestinal fluids, most likely due to a digestion of the formulation, the CBD amount increased along with the increasing CBD-NLC mean diameters and PdI.
To further confirm the above data, Bonferroni’s pairwise comparison of the means in terms of Z-Average (nm) and PdI was carried out and reported in
Figure 3. Samples were named after the time (hours) of incubation (i.e., 0, 2, 4, 6 h) and type of fluid investigated, namely SGF and SIF. Results are shown as mean ± standard error (
n = 3. The obtained data agreed with a high stability of CBD-NLC in SGF, without releasing CBD. By contrast, when the CBD-NLC was investigated in SIF, the mean diameters of the nanovectors increased significantly, especially after four hours (
Figure 3), reaching firstly the size of 575 nm and, successively, the size of 1490 nm after four hours, and 2000 nm after six hours (
Figure 2). In addition, a significant modification of PdI value was found only for the test performed in SIF, starting from the second hour (
Figure 3).
4. Discussion
Many phytoconstituents have impressive health properties, as nutraceuticals have a hydrophobic nature with a low water solubility and consequently scarce oral bioavailability. Indeed, oral bioavailability is determined by three fundamental steps: bioaccessibility, permeation through gut epithelial layers with consequent absorption and metabolism of phyto-constituents. Furthermore, a low bioavailability could also be related to a low stability in gastrointestinal fluid. The term bioaccessibility is related to the portion of the ingested phytoconstituent that becomes available for absorption [
38].
The use of nanocarriers as nutraceutical delivery systems has been recognized as the best platform to give concurrently the enhancement of all factors that influence their bioavailability [
23,
39]. Carrier materials and architectures, small sizes, superficial electrical charge and large interfacial surface profoundly affect protection of the nutraceuticals from external (oxygen, light, temperature, humidity, etc.) and physiological factors (pH and enzymes), improving the aqueous solubility, performance and residence time in the gastric and enteric tract [
39]. Materials are mainly represented by lipids due to their ability to solubilize the lipophilic phytoconstituents [
23,
39]. These nanocarriers can have a different behavior in the gastric and enteric tract due to their peculiar chemical composition imparting different properties, principally the physical state and the ability to form complex aggregates [
38]. In the present study, NLCs were developed because they are among the most attractive nutraceutical delivery systems; they are easy to prepare using nontoxic GRAS ingredients, they need low amounts of organic solvents and have an excellent encapsulation efficiency. NLCs are also very versatile in terms of chemical composition and architecture, assuring high protection of the nutraceuticals from external and physiological factors and optimizing the bioavailability [
23,
27,
28].
NLCs can be prepared using a mixture of solid and liquid lipids in order to enhance the EE. After administration, if the melting point is near to 37 °C, the NLCs are generally dissolved in the gastric medium. If melting point is greater than 40 °C when administered, these nanoparticles remain in a solid state but, according to the chemical composition, they can be digested in the enteric medium, releasing the nutraceuticals which leads to their absorption. If non-digestible lipids are used, the nutraceuticals cannot be absorbed regularly in the intestinal epithelial layer or evacuated through excretion [
27].
The developed CBD-loaded NLCs had a melting point greater than 40 °C and were investigated for their behavior in the presence of simulated gastric and intestinal fluids. The study evidenced that NLCs were very stable in the gastric medium, which is definitely a very important property because CBD is very unstable in acidic media.
CBD is also reported to be not stable in simulated physiological conditions (pH = 7.4, 37 °C), however the developed NLCs in the presence of the intestinal fluid were digested and formed an emulsion with droplet sizes of about 2 μm, which protected and preserved CBD chemical structure, as confirmed by the 100% recovery calculated by HPLC-DAD.
Hence, it is reported that digestion in the presence of lipase and pancreatin is strongly affected by the surfactant choice and selection of the lipid component. It is well known that medium chain glycerides are preferable, followed by long chain glycerides and surfactants, while waxes are poorly affected substrates [
37].
The above studies evidenced that the primary absorption of the developed CBD-loaded NLCs should occur in the duodenal region of the intestine passing intact through the stomach. These findings make CBD-loaded NLCs a promising formulation to deliver CBD by the oral administration route and optimize its bioaccessibility.