Radiation in Early-Stage Breast Cancer: Moving beyond an All or Nothing Approach
Abstract
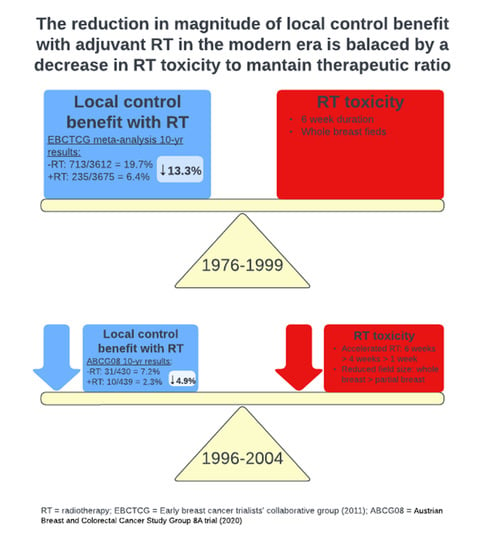
:1. Introduction
2. Selecting Patients for Radiation Omission
2.1. Age as a Selection Criterion
2.2. Combining Age and Biology
3. Emerging Data on Omission of Endocrine Therapy with Radiation Alone
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.H.; Wolmark, N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Early Breast Cancer Trialists′ Collaborative, G.; Darby, S.; McGale, P.; Correa, C.; Taylor, C.; Arriagada, R.; Clarke, M.; Cutter, D.; Davies, C.; Ewertz, M.; et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef] [Green Version]
- Fisher, B.; Bryant, J.; Dignam, J.J.; Wickerham, D.L.; Mamounas, E.P.; Fisher, E.R.; Margolese, R.G.; Nesbitt, L.; Paik, S.; Pisansky, T.M.; et al. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J. Clin. Oncol. 2002, 20, 4141–4149. [Google Scholar] [CrossRef] [PubMed]
- Early Breast Cancer Trialists′ Collaborative, G. Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet 2015, 386, 1341–1352. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists′ Collaborative, G.; Davies, C.; Godwin, J.; Gray, R.; Clarke, M.; Cutter, D.; Darby, S.; McGale, P.; Pan, H.C.; Taylor, C.; et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet 2011, 378, 771–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fyles, A.W.; McCready, D.R.; Manchul, L.A.; Trudeau, M.E.; Merante, P.; Pintilie, M.; Weir, L.M.; Olivotto, I.A. Tamoxifen with or without breast irradiation in women 50 years of age or older with early breast cancer. N. Engl. J. Med. 2004, 351, 963–970. [Google Scholar] [CrossRef]
- Fyles, A.; Manchul, L.; McCready, D.; Trudeau, M.; Olsson, S. Radiation for early breast cancer: Is less more? Discov. Med. 2005, 5, 55–57. [Google Scholar]
- Hughes, K.S.; Schnaper, L.A.; Bellon, J.R.; Cirrincione, C.T.; Berry, D.A.; McCormick, B.; Muss, H.B.; Smith, B.L.; Hudis, C.A.; Winer, E.P.; et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: Long-term follow-up of CALGB 9343. J. Clin. Oncol. 2013, 31, 2382–2387. [Google Scholar] [CrossRef] [Green Version]
- Kunkler, I.H.; Williams, L.J.; Jack, W.J.; Cameron, D.A.; Dixon, J.M.; on behalf of the PRIME II investigators. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): A randomised controlled trial. Lancet Oncol. 2015, 16, 266–273. [Google Scholar] [CrossRef]
- Arvold, N.D.; Taghian, A.G.; Niemierko, A.; Abi Raad, R.F.; Sreedhara, M.; Nguyen, P.L.; Bellon, J.R.; Wong, J.S.; Smith, B.L.; Harris, J.R. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J. Clin. Oncol. 2011, 29, 3885–3891. [Google Scholar] [CrossRef]
- Liu, F.F.; Shi, W.; Done, S.J.; Miller, N.; Pintilie, M.; Voduc, D.; Nielsen, T.O.; Nofech-Mozes, S.; Chang, M.C.; Whelan, T.J.; et al. Identification of a Low-Risk Luminal A Breast Cancer Cohort That May Not Benefit From Breast Radiotherapy. J. Clin. Oncol. 2015, 33, 2035–2040. [Google Scholar] [CrossRef]
- Coles, C.E.; Griffin, C.L.; Kirby, A.M.; Titley, J.; Agrawal, R.K.; Alhasso, A.; Bhattacharya, I.S.; Brunt, A.M.; Ciurlionis, L.; Chan, C.; et al. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet 2017, 390, 1048–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faverly, D.R.; Hendriks, J.H.; Holland, R. Breast carcinomas of limited extent: Frequency, radiologic-pathologic characteristics, and surgical margin requirements. Cancer 2001, 91, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Vicini, F.A.; Kestin, L.L.; Goldstein, N.S. Defining the clinical target volume for patients with early-stage breast cancer treated with lumpectomy and accelerated partial breast irradiation: A pathologic analysis. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 722–730. [Google Scholar] [CrossRef]
- Sanders, M.E.; Scroggins, T.; Ampil, F.L.; Li, B.D. Accelerated partial breast irradiation in early-stage breast cancer. J. Clin. Oncol. 2007, 25, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Brunt, A.M.; Wheatley, D.; Yarnold, J.; Somaiah, N.; Kelly, S.; Harnett, A.; Coles, C.; Goodman, A.; Bahl, A.; Churn, M.; et al. Acute skin toxicity associated with a 1-week schedule of whole breast radiotherapy compared with a standard 3-week regimen delivered in the UK FAST-Forward Trial. Radiother. Oncol. 2016, 120, 114–118. [Google Scholar] [CrossRef] [Green Version]
- Murray Brunt, A.; Haviland, J.S.; Wheatley, D.A.; Sydenham, M.A.; Alhasso, A.; Bloomfield, D.J.; Chan, C.; Churn, M.; Cleator, S.; Coles, C.E.; et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 2020, 395, 1613–1626. [Google Scholar] [CrossRef]
- Polgar, C.; Fodor, J.; Major, T.; Sulyok, Z.; Kasler, M. Breast-conserving therapy with partial or whole breast irradiation: Ten-year results of the Budapest randomized trial. Radiother. Oncol. 2013, 108, 197–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, N.; Sanz, X.; Dengra, J.; Foro, P.; Membrive, I.; Reig, A.; Quera, J.; Fernandez-Velilla, E.; Pera, O.; Lio, J.; et al. Five-year outcomes, cosmesis, and toxicity with 3-dimensional conformal external beam radiation therapy to deliver accelerated partial breast irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 1051–1057. [Google Scholar] [CrossRef]
- Strnad, V.; Ott, O.J.; Hildebrandt, G.; Kauer-Dorner, D.; Knauerhase, H.; Major, T.; Lyczek, J.; Guinot, J.L.; Dunst, J.; Gutierrez Miguelez, C.; et al. 5-year results of accelerated partial breast irradiation using sole interstitial multicatheter brachytherapy versus whole-breast irradiation with boost after breast-conserving surgery for low-risk invasive and in-situ carcinoma of the female breast: A randomised, phase 3, non-inferiority trial. Lancet 2016, 387, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Vicini, F.A.; Cecchini, R.S.; White, J.R.; Arthur, D.W.; Julian, T.B.; Rabinovitch, R.A.; Kuske, R.R.; Ganz, P.A.; Parda, D.S.; Scheier, M.F.; et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: A randomised, phase 3, equivalence trial. Lancet 2019, 394, 2155–2164. [Google Scholar] [CrossRef] [PubMed]
- Whelan, T.J.; Julian, J.A.; Berrang, T.S.; Kim, D.H.; Germain, I.; Nichol, A.M.; Akra, M.; Lavertu, S.; Germain, F.; Fyles, A.; et al. External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): A randomised controlled trial. Lancet 2019, 394, 2165–2172. [Google Scholar] [CrossRef]
- Meattini, I.; Marrazzo, L.; Saieva, C.; Desideri, I.; Scotti, V.; Simontacchi, G.; Bonomo, P.; Greto, D.; Mangoni, M.; Scoccianti, S.; et al. Accelerated Partial-Breast Irradiation Compared with Whole-Breast Irradiation for Early Breast Cancer: Long-Term Results of the Randomized Phase III APBI-IMRT-Florence Trial. J. Clin. Oncol. 2020, 38, 4175–4183. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.A.; Xiao, J.; Oh, C.; Taneja, S.; Barbee, D.; Maisonet, O.; Huppert, N.; Perez, C.; Gerber, N.K. Five-Fraction Prone Accelerated Partial Breast Irradiation: Long-Term Oncologic, Dosimetric, and Cosmetic Outcome. Pract. Radiat. Oncol. 2022, 12, 106–112. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Fang, F.; Varnum, C.; Eriksson, M.; Hall, P.; Czene, K. Predictors of Discontinuation of Adjuvant Hormone Therapy in Patients with Breast Cancer. J. Clin. Oncol. 2015, 33, 2262–2269. [Google Scholar] [CrossRef]
- Hershman, D.L.; Shao, T.; Kushi, L.H.; Buono, D.; Tsai, W.Y.; Fehrenbacher, L.; Kwan, M.; Gomez, S.L.; Neugut, A.I. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res. Treat. 2011, 126, 529–537. [Google Scholar] [CrossRef] [Green Version]
- Huiart, L.; Ferdynus, C.; Giorgi, R. A meta-regression analysis of the available data on adherence to adjuvant hormonal therapy in breast cancer: Summarizing the data for clinicians. Breast Cancer Res. Treat. 2013, 138, 325–328. [Google Scholar] [CrossRef]
- Gerber, N.K.; Shao, H.; Chadha, M.; Deb, P.; Gold, H.T. Radiation Without Endocrine Therapy in Older Women with Stage I Estrogen-Receptor-Positive Breast Cancer is Not Associated with a Higher Risk of Second Breast Cancer Events. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 40–51. [Google Scholar] [CrossRef]
- Esserman, L.J.; Yau, C.; Thompson, C.K.; van’t Veer, L.J.; Borowsky, A.D.; Hoadley, K.A.; Tobin, N.P.; Nordenskjold, B.; Fornander, T.; Stal, O.; et al. Use of Molecular Tools to Identify Patients with Indolent Breast Cancers with Ultralow Risk Over 2 Decades. JAMA Oncol. 2017, 3, 1503–1510. [Google Scholar] [CrossRef]
- Cardoso, F.; van’t Veer, L.J.; Bogaerts, J.; Slaets, L.; Viale, G.; Delaloge, S.; Pierga, J.Y.; Brain, E.; Causeret, S.; DeLorenzi, M.; et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N. Engl. J. Med. 2016, 375, 717–729. [Google Scholar] [CrossRef] [Green Version]
- Lopes Cardozo, J.M.N.; Drukker, C.A.; Rutgers, E.J.T.; Schmidt, M.K.; Glas, A.M.; Witteveen, A.; Cardoso, F.; Piccart, M.; Esserman, L.J.; Poncet, C.; et al. Outcome of Patients with an Ultralow-Risk 70-Gene Signature in the MINDACT Trial. J. Clin. Oncol. 2022, 40, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Schnabel, C.A.; Schroeder, B.E.; Jerevall, P.L.; Jankowitz, R.C.; Fornander, T.; Stal, O.; Brufsky, A.M.; Sgroi, D.; Erlander, M.G. Breast cancer index identifies early-stage estrogen receptor-positive breast cancer patients at risk for early- and late-distant recurrence. Clin. Cancer Res. 2013, 19, 4196–4205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, M.C.; Vicini, F.; Al-Hilli, Z.; Chadha, M.; Pierce, L.; Recht, A.; Hayman, J.; Thaker, N.; Khan, A.J.; Keisch, M.; et al. Cost-effectiveness analysis of endocrine therapy alone versus partial-breast irradiation alone versus combined treatment for low-risk hormone-positive early-stage breast cancer in women aged 70 years or older. Breast Cancer Res. Treat. 2020, 182, 355–365. [Google Scholar] [CrossRef] [PubMed]
| Author/Study | Dates of Accrual | Final Date of Publication | Patients | Randomization | Radiotherapy | Age | Tumor Subtype | Tumor Size | IBTR | Notable Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Fisher et al. (NSABP B21) | 1989–1998 | 2002 | 1009 patients’ status post lumpectomy | TAM vs. XRT vs. XRT + TAM | 50 Gy in 2 Gy fractions, optional boost (median dose 10 Gy) | 20% <50 years old; 16% >70 years old | 56.7% ER+ | All patients ≤1 cm (27.7% ≤ 5 mm) | At 8 years, TAM: 16.5%; XRT: 9.3%; XRT + TAM: 2.8% | Significant reduction in contralateral breast cancer with tamoxifen; OS in 3 groups were equivalent at 93–94% |
| Fyles et al. | 1992–2000 | 2004 | 769 women with pT1-2N0 breast cancer status post-lumpectomy | TAM vs. XRT + TAM | 40 Gy in 2.5 Gy fractions, 12.5 Gy boost in 2.5 Gy fractions, 16 treatments (3–4 weeks) | All patients ≥50 years old; 42% ≥70 years old | 80.8% HR+ | 34.7% ≤ 10 mm | At 8 years, TAM: 17.6% vs. TAM + XRT: 3.5% | Planned subgroup analysis of T1, HR+ (n = 611) with 8-year IBTR of 15.2% vs. 3.6%. Unplanned subgroup analysis of women >60, tumors <1 cm (n = 193), HR+ with 8 year IBTR 3.6% vs. 0% |
| Hughes et al. (CALGB 9343) | 1994–1999 | 2013 | 636 women with T1N0M0 breast cancer status post lumpectomy | TAM vs. XRT + TAM | 45 Gy in 1.8 Gy fractions, electron boost of 14 Gy in 2 Gy fractions, 32 treatments (6.5 weeks) | All patients ≥70; 55% ≥75 | 99% ER+ | 98% ≤ 20 mm | At 10 years, 98% in XRT + TAM vs. 90% in TAM arm were free from LRR | LRR benefit did not translate into an advantage in OS, DFS, or breast preservation |
| Kunkler et al. (PRIME II) | 2003–2009 | 2015 | 1326 women with node-negative breast cancer status post lumpectomy | XRT vs. no XRT | 40–50 Gy in 2.66–2.00 Gy per fraction, allowance of 10–15 Gy electron boost or iridium implant, 15–25 treatments | All patients ≥65 | All patients ER+, PR+, or both. | Tumors up to 3 cm (39.4% ≤10 mm) | At 5 years, XRT: 1.3% vs. no XRT: 4.1%; at 10 years, XRT: 0.9% vs no XRT: 9.8% | There were no differences in regional recurrence, contralateral breast cancer, or distant metastases. Most patients dying of causes unrelated to breast cancer. |
| Trial | Clinicaltrials.gov Identifier | Study Start Date | Estimated Study Completion Date | Study Design | Age | Molecular Subtype | Grade | Biological Selection | Target Accrual | Recruitment Status | Preliminary Results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| LUMINA | NCT01791829 | July 2013 | December 2024 | Phase II, single-arm observational | >50 years | ER and PR+ in ≥10%; HER2- on IHC (0 or 1+) or FISH | Grade 1 or 2 | Luminal A subtype by IHC | 500 | Active, not recruiting | ASCO 2022: at 5 years, 10 local recurrences (2.3%) and 8 contralateral breast cancer events (1.9%); OS 97.2%, DFS 89.9%, RFS 97.3% |
| IDEA | NCT02400190 | March 2015 | March 2026 | Phase II, single-arm observational | 50–69 years | ER+, PR+, Her2- using the current College of American Pathologists guidelines | - | Oncotype DX RS ≤18 | 250 | Active, not recruiting | - |
| PRECISION | NCT0265375 | May 2016 | December 2025 | Phase II, single-arm observational | 50–75 years | ER+ (≥ 10%) or PR+, HER2- | Grade 1 or 2 | Prosigna PAM-50, ROR ≤40 | 671 screened, 382 study population | Active, not recruiting | - |
| EXPERT | NCT02889874 | August 2017 | December 2023 | Phase III Randomized trial RT vs observation | ≥50 years | ER and PR+ in ≥10%; HER2- on IHC (0 or 1+) or FISH | Grade 1 or 2 | Prosigna PAM-50, ROR ≤60 | 1167 | Active, recruiting | - |
| DEBRA | NCT04852887 | June 2021 | July 2024 | Phase III Randomized trial RT vs observation | 50–<70 years | ER≥1%, PR+, Her2- using ASCO/CAP Guideline Recommendations | - | Oncotype DX RS ≤18 | 1670 | Active, recruiting | - |
| Author/Study | Country | Dates of Accrual | Final Date of Publication | Patients | PBI | WBI | Boost in WBI Arm | Follow up | IBTR | Toxicity and Cosmesis |
|---|---|---|---|---|---|---|---|---|---|---|
| Polgar et al. | Hungary | 2006–2019 | 2013 | 258 patients with pT1 (≤ 2 cm), pN0–1 mi, negative margins, age >40 | 36.4 Gy/7 fx (HDR) or 50 Gy/25 fx (electrons) | 50 Gy/ 25 fx | 16 Gy electrons (0.8%) | 10.2 years | APBI: 5.9% vs. WBI: 5.1% | APBI had higher excellent-good cosmetic score (81% vs. 63%) |
| Rodriguez et al. | Barcelona | N/A | 2013 | 102 patients with pT1–2 (≤3 cm), pN0, margins ≥ 2 mm, age ≥ 60 | 37.5 Gy/10 fx BID (3D-CRT) | 48 Gy/24 fx | 10 Gy (66.0%) | 5 years | APBI: 0% vs. WBI: 0% | No difference in late skin toxicity or cosmesis |
| Strnad et al. (GEC-ESTRO) | Austria, Czech Republic, Germany, Hungary, Poland, Spain, and Switzerland | 2004–2009 | 2016 | 1184 patients with pT1–2 (<3 cm), pN0–1 mi, margins ≥ 2 mm, age ≥ 40 | 32 Gy/8 fx or 30.2 Gy/7 fx (HDR) or 50 Gy (PDR) | 50 Gy/25 fx | 10 Gy electrons (100.0%) | 6.6 years | APBI: 1.4% vs. WBI: 0.92% (p=0.42) | Significantly lower grade 2+ late skin effects with APBI |
| Coles et al. (IMPORT LOW) | United Kingdom | 2007–2016 | 2017 | 2018 patients with pT1-2 (<3 cm), N0–1, margins ≥ 2 mm, age ≥ 50 | 40 Gy/15 fx | 40 Gy/15 fx vs 36 Gy + 40 Gy boost | Simultaneous integrated boost in 36 Gy+40 Gy arm | 6 years | PBI: 0.5% vs. WBI: 1.1% vs. reduced dose WBI + boost: 0.2% | Reduced toxicity in both experimental arms |
| Vicini et al. (NSABP B-39) | USA, Canada, Ireland, and Israel | 2005–2018 | 2019 | 4216 patients with pT1–2 (<3 cm), pN0–1 (1–3), negative margins, age ≥ 18 | 38.5 Gy/10 fx BID (3D-CRT) or 34 Gy/10 fx (HDR) | 50 Gy/ 25 fx WBI | 10–16 Gy (80%) | 10.2 years | APBI: 4.6% vs. WBI: 3.9% (HR did not meet criteria for equivalence) | In non-chemotherapy treated patients, APBI had slightly poorer cosmesis at 3 years |
| Whelan et al. (RAPID) | Canada | 2006–2918 | 2019 | 2135 patients with pT1–2 (≤ 2 cm), pN0–1 mic, negative margins, age ≥ 40 | 38.5 Gy/10 fx BID (3D-CRT) | 42.5 Gy/ 16 fx (82%), 50 Gy/25 fx (18%) | 10 Gy (21%) | 8.6 years | APBI: 3% vs. WBI: 2.8% (HR met criteria for equivalence) | APBI had less acute and more late toxicity (grade 2+), similar patient-rated cosmetic outcome |
| Livi et al. (Florence) | Italy | 2005–2013 | 2020 | 520 patients with pT1–2 (<2.5 cm), negative margins, age > 40 | 30 Gy/5 fx once-daily, non-consecutive days | 50 Gy / 25 fx | 10 Gy electrons (100%) | 10.7 years | APBI: 3.7% vs WBI: 2.5% (p = 0.40) | APBI had less acute and late toxicity and improved patient and physician rated cosmetic outcome |
| Vaidya et al. (TARGIT) | United Kingdom, Europe, Australia, the United States, and Canada | 2000–2012 | 2020 | 2298 patients, <3.5 cm, cN0-N1, age >45 | 20 Gy IORT | 3–6 weeks EBRT | optional boost | 5 years | IORT: 2.11% vs WBI: 0.95% | Grade 3 or 4 radiotherapy toxicity was significantly reduced with TARGIT |
| Orrechia et al. (ELIOT) | Italy | 2000–2007 | 2021 | 1305 patients, <25 mm, cN0, age 48–75 years | 21 Gy IORT | 50 Gy/ 25 fx | 10 Gy (100%) | 12.4 years | IORT: 11% vs WBI: 2% | - |
| ASTRO APBI Guidelines | pT1 (≤ 2 cm), pN0–1 mi, margins ≥ 2 mm, age ≥ 50; DCIS: screen-detected, 1–2 nuclear grade, ≤2.5 cm size, margins ≥ 3 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purswani, J.M.; Hardy-Abeloos, C.; Perez, C.A.; Kwa, M.J.; Chadha, M.; Gerber, N.K. Radiation in Early-Stage Breast Cancer: Moving beyond an All or Nothing Approach. Curr. Oncol. 2023, 30, 184-195. https://doi.org/10.3390/curroncol30010015
Purswani JM, Hardy-Abeloos C, Perez CA, Kwa MJ, Chadha M, Gerber NK. Radiation in Early-Stage Breast Cancer: Moving beyond an All or Nothing Approach. Current Oncology. 2023; 30(1):184-195. https://doi.org/10.3390/curroncol30010015
Chicago/Turabian StylePurswani, Juhi M., Camille Hardy-Abeloos, Carmen A. Perez, Maryann J. Kwa, Manjeet Chadha, and Naamit K. Gerber. 2023. "Radiation in Early-Stage Breast Cancer: Moving beyond an All or Nothing Approach" Current Oncology 30, no. 1: 184-195. https://doi.org/10.3390/curroncol30010015
APA StylePurswani, J. M., Hardy-Abeloos, C., Perez, C. A., Kwa, M. J., Chadha, M., & Gerber, N. K. (2023). Radiation in Early-Stage Breast Cancer: Moving beyond an All or Nothing Approach. Current Oncology, 30(1), 184-195. https://doi.org/10.3390/curroncol30010015