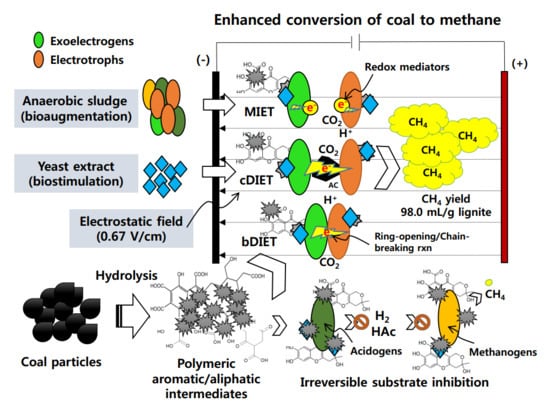
Contribution of Yeast Extract, Activated Carbon, and an Electrostatic Field to Interspecies Electron Transfer for the Bioelectrochemical Conversion of Coal to Methane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coal, Yeast Extract, Anaerobic Medium, and Seed Sludge
2.2. Experimental Setup for Bioelectrochemical Methane Conversion of Coal
2.3. Analysis and Calculations
2.4. Microbial Community
3. Results and Discussion
3.1. Production of Methane from Coal
3.2. Intermediates of Coal Degradation
3.3. Methane Production from the Intermediates of Coal Degradation
3.4. Cyclic Voltammetry and EIS
3.5. Microbial Communities
3.6. Implications
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, J.C.; Liu, Z.S.; Huang, J.S. Emission characteristics of coal combustion in different O2/N2, O2/CO2 and O2/RFG atmosphere. J. Hazard. Mater. 2007, 142, 266–271. [Google Scholar] [CrossRef]
- Dmitrienko, M.A.; Strizhak, P.A. Environmentally and economically efficient utilization of coal processing waste. Sci. Total Environ. 2017, 598, 21–27. [Google Scholar] [CrossRef]
- United States Energy Information Administration (USEIA), Carbon Dioxide Emission Coefficients: Carbon Dioxide Emissions Coefficients by Fuel. Available online: https://www.eia.gov/environment/emissions/co2_vol_mass.php (accessed on 16 December 2018).
- Greiner, P.T.; York, R.; McGee, J.A. Snakes in The Greenhouse: Does increased natural gas use reduce carbon dioxide emissions from coal consumption? Energy Res. Soc. Sci. 2018, 38, 53–57. [Google Scholar] [CrossRef]
- Hultman, N.; Rebois, D.; Scholten, M.; Ramig, C. The greenhouse impact of unconventional gas for electricity generation. Environ. Res. Lett. 2011, 6, 044008. [Google Scholar] [CrossRef]
- Ritter, D.; Vinson, D.; Barnhart, E.; Akob, D.M.; Fields, M.W.; Cunningham, A.B.; Orem, W.; McIntosh, J.C. Enhanced microbial coalbed methane generation: A review of research, commercial activity, and remaining challenges. Int. J. Coal Geol. 2015, 146, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Strapoc, D.; Mastalerz, M.; Dawson, K.; Macalady, J.; Callaghan, A.V.; Wawrik, B.; Turich, C.; Ashby, M. Biogeochemistry of Microbial Coal-Bed Methane. Annu. Rev. Earth Planet. Sci. 2011, 39, 617–656. [Google Scholar] [CrossRef]
- Park, S.Y.; Liang, Y. Biogenic methane production from coal: A review on recent research and development on microbially enhanced coalbed methane (MECBM). Fuel 2016, 166, 258–267. [Google Scholar] [CrossRef] [Green Version]
- Piao, D.M.; Song, Y.C.; Kim, D.H. Bioelectrochemical Enhancement of Biogenic Methane Conversion of Coal. Energies 2018, 11, 2577. [Google Scholar] [CrossRef]
- Bao, Y.; Huang, H.; He, D.; Ju, Y.; Qi, Y. Microbial enhancing coal-bed methane generation potential, constraints and mechanism. J. Nat. Gas Sci. Eng. 2016, 35, 68–78. [Google Scholar] [CrossRef]
- Bucha, M.; Jędryseka, M.O.; Kufkab, D.; Pleśniaka, L.; Marynowskic, L.; Kubiakd, K.; Błaszczyke, M. Methanogenic fermentation of lignite with carbon-bearing additives, inferred from stable carbon and hydrogen isotopes. Int. J. Coal Geol. 2018, 186, 65–79. [Google Scholar] [CrossRef]
- Senthamaraikkannan, G.; Gates, I.; Prasad, V. Multiphase reactive-transport simulations for estimation and robust optimization of the field scale production of microbially enhanced coalbed methane. Chem. Eng. Sci. 2016, 149, 63–77. [Google Scholar] [CrossRef]
- Feng, Q.; Song, Y.C.; Kim, D.H.; Kim, M.S.; Kim, D.H. Influence of the temperature and hydraulic retention time in bioelectrochemical anaerobic digestion of sewage sludge. Int. J. Hydrog. Energy 2019, 44, 2170–2179. [Google Scholar] [CrossRef]
- Zheng, H.; Chen, T.; Rudolph, V.; Golding, S.D. Biogenic methane production from Bowen Basin coal waste materials. Int. J. Coal Geol. 2017, 169, 22–27. [Google Scholar] [CrossRef]
- Wang, B.; Tai, C.; Wu, L.; Chen, L.; Liu, J.M.; Hu, B.; Song, D. Methane production from lignite through the combined effects of exogenous aerobic and anaerobic microflora. Int. J. Coal Geol. 2017, 173, 84–93. [Google Scholar] [CrossRef]
- Fuertez, J.; Nguyen, V.; McLennan, J.D.; Adams, D.J.; Han, K.B.; Sparks, T.D. Optimization of biogenic methane production from coal. Int. J. Coal Geol. 2017, 183, 14–24. [Google Scholar] [CrossRef]
- Colosimo, F.; Thomas, R.; Lloyd, J.R.; Taylor, K.G.; Boothman, C.; Smith, A.D.; Lord, R.; Kalin, R.M. Biogenic methane in shale gas and coal bed methane: A review of current knowledge and gaps. Int. J. Coal Geol. 2016, 165, 106–120. [Google Scholar] [CrossRef] [Green Version]
- Orem, W.H.; Voytek, M.A.; Jones, E.J.; Lerch, H.E.; Bates, A.L.; Corum, M.D.; Warwick, P.D.; Clark, A.C. Organic intermediates in the anaerobic biodegradation of coal to methane under laboratory conditions. Org. Geochem. 2010, 41, 997–1000. [Google Scholar] [CrossRef]
- Davis, K.J.; Lu, S.; Barnhart, E.P.; Parker, A.E.; Fields, M.W.; Gerlach, R. Type and amount of organic amendments affect enhanced biogenic methane production from coal and microbial community structure. Fuel 2018, 211, 600–608. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, S.B.; Sharma, R.; Chaudhary, P.; Pandey, A.K.; Ansari, R.; Vasudevan, V.; Arora, A.; Singh, S.; Saha, S.; et al. Enhanced biodegradation of PAHs by microbial consortium with different amendment and their fate in in-situ condition. J. Environ. Manag. 2016, 181, 728–736. [Google Scholar] [CrossRef]
- Dubé, C.D.; Guiot, S.R. Direct interspecies electron transfers in anaerobic diges-tion: A review. Biogas Sci. Technol. 2015, 151, 101–115. [Google Scholar] [CrossRef]
- Feng, Q.; Song, Y.C.; Ahn, Y. Electroactive microorganisms in bulk solution contribute significantly to methane production in bioelectrochemical anaerobic reactor. Bioresour. Technol. 2018, 259, 119–127. [Google Scholar] [CrossRef]
- Rotaru, A.E.; Shrestha, P.M.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Zengler, K.; Wardman, C.; Nevin, K.P.; Lovley, D.R. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 2014, 7, 408–415. [Google Scholar] [CrossRef]
- Jones, E.J.P.; Voytek, M.A.; Corum, M.D.; Orem, W.H. Stimulation of Methane Generation from Nonproductive Coal by Addition of Nutrients or a Microbial Consortium. Appl. Environ. Microbiol. 2010, 76, 7013–7022. [Google Scholar] [CrossRef] [Green Version]
- Baek, G.; Kim, J.; Kim, J.; Lee, C. Role and Potential of Direct Interspecies Electron Transfer in Anaerobic Digestion. Energies 2018, 11, 107. [Google Scholar] [CrossRef]
- Park, J.H.; Kang, H.J.; Park, K.H.; Park, H.D. Direct interspecies electron transfer via conductive materials: A perspective for anaerobic digestion applications. Bioresour. Technol. 2018, 254, 300–311. [Google Scholar] [CrossRef]
- Baxter, T.E.; Rexing, D.J. Physical and aggregate properties. Standard Methods for the Examination of Water and Wastewater, 21st ed.; Eaton, A.D., Clesceri, L.S., Rice, E.W., Greenberg, A.E., Eds.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2005; pp. 2–55. [Google Scholar]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation−Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Chun, G.; Bae, D.; Nickens, K.; O’Brien, T.J.; Patierno, S.R.; Ceryak, S. Polo-like kinase 1 enhances survival and mutagenesis after genotoxic stress in normal cells through cell cycle checkpoint bypass. Carcinogenesis 2010, 31, 785–793. [Google Scholar] [CrossRef] [Green Version]
- Girguis, P.R.; Orphan, V.J.; Hallam, S.J.; DeLong, E.F. Growth and Methane Oxidation Rates of Anaerobic Methanotrophic Archaea in a Continuous-Flow Bioreactor. Appl. Eenviron. Microbiol. 2003, 69, 5472–5482. [Google Scholar] [CrossRef] [Green Version]
- Kurth, J.M.; Smit, N.T.; Berger, S.; Schouten, S.; Jetten, M.S.M.; Welte, C.U. Anaerobic methanotrophic archaea of the ANME-2d clade feature lipid composition that differs from other ANME archaea. FEMS Microbiol. Ecol. 2019, 95, fiz082. [Google Scholar] [CrossRef]
- Timmers, P.H.A.; Welte, C.U.; Koehorst, J.J.; Plugge, C.M.; Jetten, M.S.M.; Stams, A.J.M. Reverse Methanogenesis and Respiration in Methanotrophic Archaea. Archaea 2017, 2017, 1654237. [Google Scholar] [CrossRef]
- Pandey, V.C.; Singh, J.S.; Singh, D.P.; Singh, R.P. Methanotrophs: Promising bacteria for environmental remediation. Int. J. Environ. Sci. Technol. 2014, 11, 241–250. [Google Scholar] [CrossRef]
- Chen, S.; Rotaru, A.E.; Shrestha, P.M.; Malvankar, N.S.; Liu, F.; Fan, W.; Nevin, K.P.; Lovley, D.R. Promoting Interspecies Electron Transfer with Biochar. Sci. Rep. 2014, 4, 5019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Rotaru, A.E.; Shrestha, P.M.; Malvankar, N.S.; Nevin, K.P.; Lovley, D.R. Promoting direct interspecies electron transfer with activated carbon. Energy Environ. Sci. 2012, 5, 8982–8989. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Ye, L.; Jin, J.; Chen, H.; Xu, X.; Zhu, L. Magnetite nanoparticles enhance the performance of a combined bioelectrode-UASB reactor for reductive transformation of 2, 4-dichloronitrobenzene. Sci. Rep. 2017, 7, 10319. [Google Scholar] [CrossRef]
- Nzila, A. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons under anaerobic conditions: Overview of studies, proposed pathways and future perspectives. Environ. Pollut. 2018, 239, 788–802. [Google Scholar] [CrossRef]
- Saánchez, N.M.; Klerk, A.D. Oxidative ring-opening of aromatics: Decomposition of biphenyl carboxylic acids and zinc biphenyl carboxylates. Energy Fuels 2015, 29, 7910–7922. [Google Scholar] [CrossRef]
- Stempfle, F.; Ortmann, P.; Mecking, S. Long-Chain Aliphatic Polymers to Bridge the Gap between Semicrystalline Polyolefins and Traditional Polycondensates. Chem. Rev. 2016, 116, 4597–4641. [Google Scholar] [CrossRef]
- Song, Y.C.; Joicy, A.; Jang, S.H. Direct interspecies electron transfer in bulk solution significantly contributes to bioelectrochemical nitrogen removal. Int. J. Hydrog. Energy 2019, 44, 2180–2190. [Google Scholar] [CrossRef]
- Harnisch, F.; Freguia, S. A basic tutorial on cyclic voltammetry for the investigation of electroactive microbial biofilms. Asian J. Chem. 2012, 7, 466–475. [Google Scholar] [CrossRef]
- Lim, S.S.; Yu, E.H.; Daud, W.R.W.; Kim, B.H.; Scott, K. Bioanode as a limiting factor to biocathode performance in microbial electrolysis cells. Bioresour. Technol. 2017, 238, 313–324. [Google Scholar] [CrossRef]
- Leang, C.; Qian, X.; Mester, T.; Lovley, D.R. Alignment of the c-Type Cytochrome OmcS along Pili of Geobacter sulfurreducens. Appl. Environ. Microbiol. 2010, 76, 4080–4084. [Google Scholar] [CrossRef]
- Lovley, D.R. Reach out and touch someone: Potential impact of DIET (direct interspecies energy transfer) on anaerobic biogeochemistry, bioremediation, and bioenergy. Rev. Environ. Sci. Biotechnol. 2011, 10, 101–105. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Wang, L.; Quan, X. Potential for direct interspecies electron transfer in an electric-anaerobic system to increase methane production from sludge digestion. Sci. Rep. 2015, 5, 11094. [Google Scholar] [CrossRef]
- Riviere, D.; Desvignes, V.; Pelletier, E.; Chaussonnerie, S.; Guermazi, S.; Weissenbach, J.; Li, T.; Camacho, P.; Sghir, A. Towards the definition of a core of microorganisms involved in anaerobic digestion of sludge. ISME J. 2009, 3, 700–714. [Google Scholar] [CrossRef] [Green Version]
- Tourlousse, D.M.; Honda, T.; Matsuura, N.; Ohashi, A.; Tonouchi, A.; Sekiguchi, Y. Draft Genome Sequence of Bacteroidales Strain 6E, Isolated from a Rice Paddy Field in Japan. Genome Announc. 2015, 3, e01167-15. [Google Scholar] [CrossRef]
- Feng, Q.; Song, Y.C.; Bae, B.U. Influence of applied voltage on the performance of bioelectrochemical anaerobic digestion of sewage sludge and planktonic microbial communities at ambient temperature. Bioresour. Technol. 2016, 220, 500–508. [Google Scholar] [CrossRef]
- Pelletier, E.; Kreimeyer, A.; Bocs, S.; Rouy, Z.; Gyapay, G.; Chouari, R.; Riviere, D.; Ganesan, A.; Daegelen, P.; Sghir, A.; et al. ‘‘Candidatus Cloacamonas acidaminovorans”: Genome sequence reconstruction provides a first glimpse of a new bacterial division. J. Bacteriol. 2008, 190, 2572–2579. [Google Scholar] [CrossRef]
- Macalady, J.L.; Lyon, E.H.; Koffman, B.; Albertson, L.K.; Meyer, K.; Galdenzi, S.; Mariani, S. Dominant microbial populations in limestone-corroding stream biofilms, Frasassi cave system, Italy. Appl. Environ. Microbiol. 2006, 72, 5596–5609. [Google Scholar] [CrossRef]
- Li, D.; Qi, R.; Yang, M.; Zhang, Y.; Yu, T. Bacterial community characteristics under long-term antibiotic selection pressures. Water Res. 2011, 45, 6063–6073. [Google Scholar] [CrossRef]
- Patel, G.B. Characterization and nutritional properties of Methanothrix concilii sp. nov., a mesophilic, aceticlastic methanogen. Can. J. Microbiol. 1984, 30, 1383–1396. [Google Scholar] [CrossRef]
- Patel, G.B.; Dennis Sprott, G. Methanosaeta concilii gen. nov. sp. nov. (“Methanothrix concilii”) and Methanosaeta thermoacetophila nom. rev., comb. nov. Int. J. Syst. Bacteriol. 1990, 40, 79–82. [Google Scholar] [CrossRef]
- Dridi, B.; Fardeau, M.L.; Ollivier, B.; Raoult, D.; Drancourt, M. Methanomassiliicoccus luminyensis gen. nov., sp. nov., a methanogenic archaeon isolated from human faeces. Int. J. Syst. Evolut. Microbiol. 2012, 62, 1902–1907. [Google Scholar] [CrossRef] [Green Version]
- Borrel, G.; Harris, H.M.B.; Parisot, N.; Gaci, N.; Tottey, W.; Mihajlovski, A.; Deane, J.; Gribaldo, S.; Bardot, O.; Peyretaillade, E.; et al. Genome Sequence of “Candidatus Methanomassiliicoccus intestinalis” Issoire-Mx1, a Third Thermoplasmatales-Related Methanogenic Archaeon from Human Feces. Genome Announc. 2013, 1, e00453-13. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Y. Evaluating approaches for sustaining methane production from coal through biogasification. Fuel 2017, 202, 233–240. [Google Scholar] [CrossRef] [Green Version]
- Nobu, M.K.; Narihiro, T.; Kuroda, K.; Mei, R.; Liu, W.T. Chasing the elusive Euryarchaeota class WSA2: Genomes reveal a uniquely fastidious methylreducing methanogen. ISME J. 2016, 10, 2478–2487. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Y.; Harpalani, S. Optimization of methane production from bituminous coal through Biogasification. Appl. Energy 2016, 183, 31–42. [Google Scholar] [CrossRef]
- Yi, L.; Feng, J.; Qin, Y.H.; Li, W.Y. Prediction of elemental composition of coal using proximate analysis. Fuel 2017, 193, 315–321. [Google Scholar] [CrossRef]
- Song, Y.C.; Feng, Q.; Ahn, Y. Performance of the Bio-electrochemical Anaerobic Digestion of Sewage Sludge at Different Hydraulic Retention Times. Energy Fuels 2016, 30, 352–359. [Google Scholar] [CrossRef]
- Nzila, A. Current status of the degradation of aliphatic and aromatic petroleum hydrocarbons by thermophilic microbes and future perspectives. Int. J. Environ. Res. Public Health 2018, 15, 2782. [Google Scholar] [CrossRef]






| Content | Blank | Control | AC | AE33 | AE67 |
|---|---|---|---|---|---|
| Medium (mL) | 250 | 250 | 250 | 250 | 250 |
| Sludge (mL) | 250 | 250 | 250 | 250 | 250 |
| Activated Carbon (g) | - | - | 1.50 | 1.50 | 1.50 |
| Electrostatic field (V/cm) | - | - | - | 0.33 | 0.67 |
| Content | Control | AC | AE33 | AE67 | |
|---|---|---|---|---|---|
| Coal | Cumulative CH4 (mL) | 172.2 | 158.3 | 177.9 | 218.1 |
| CH4 yield (mL/g lignite) | - | - | 12.2 | 31.2 | |
| Organic residue (mg SCOD/L) | 2686.3 | 2855.9 | 3043.5 | 3127.8 | |
| Organic residue | Cumulative CH4 (mL) | 82.4 | 49.3 | 52.4 | 171.0 |
| CH4 yield (mL/g lignite) | - | - | - | 66.8 | |
| Total CH4 yield (mL/g lignite) | - | - | 12.2 | 98.0 | |
| Electrochemical Properties | Control | AC | AE33 | AE67 | ||
|---|---|---|---|---|---|---|
| CV | Ef (vs. Ag/AgCl) | −0.25 | 0.13 | 0.17 | 0.22 | |
| Ip,ox/Ip,red (mA) | 0.58/0.64 | 0.71/1.39 | 0.87/1.19 | 1.12/1.14 | ||
| Equivalent circuit for EIS data, (L-Rs-Q|(Rct-W)) | L (Ω) | 3.33 u | 3.62 u | 3.44 u | 3.58 u | |
| Rs (Ω) | 7.86 | 3.02 | 2.42 | 2.74 | ||
| Q | Qy | 0.27 m | 94.47 u | 45.50 u | 25.29 u | |
| Qa | 0.57 | 0.83 | 0.92 | 0.94 | ||
| Rct (Ω) | 10.62 | 1.55 | 1.19 | 1.45 | ||
| W (Ω/√s) | 2.02 m | 8.40 m | 7.35 m | 8.64 m | ||
| r2 | 0.998 | 0.999 | 0.999 | 0.998 | ||
| Contents | Bacteria | Archaea | ||||||
|---|---|---|---|---|---|---|---|---|
| Control | AC | AE33 | AE67 | Control | AC | AE33 | AE67 | |
| Valid reads | 42,387 | 49,041 | 46,822 | 42,770 | 22,536 | 18,365 | 19,376 | 18,970 |
| OTUs | 1485 | 1642 | 1568 | 1535 | 90 | 207 | 228 | 153 |
| Jackknife | 1697 | 1873 | 1741 | 1757 | 96 | 219 | 236 | 168 |
| Shannon | 5.001 | 5.138 | 5.015 | 5.024 | 2.189 | 2.796 | 2.932 | 2.562 |
| Classification | Taxonomic Composition | Control (%) | AC (%) | AE33 (%) | AE67 (%) |
|---|---|---|---|---|---|
| Bacteria | |||||
| Phylum | Bacteroidetes | 44.3 | 45.6 | 44.9 | 45.1 |
| Proteobacteria | 18.0 | 24.6 | 23.3 | 23.0 | |
| Firmicutes | 10.7 | 9.8 | 9.7 | 10.4 | |
| Cloacamonas_p | 9.1 | 6.7 | 8.1 | 6.5 | |
| Others | 17.9 | 13.3 | 14.0 | 15.0 | |
| Genus | GQ396981_g | 10.1 | 10.0 | 9.2 | 9.9 |
| BBZD_g | 7.3 | 7.5 | 7.2 | 7.0 | |
| Cloacamonas | 7.4 | 6.2 | 7.6 | 6.0 | |
| DQ415754_g | 6.1 | 1.8 | 0.5 | 6.0 | |
| Porphyromonadaceae_uc | 5.3 | 5.2 | 4.7 | 5.6 | |
| Thermomonas | 2.4 | 2.0 | 1.9 | 2.9 | |
| Others | 61.5 | 67.3 | 68.9 | 62.5 | |
| Species | GQ396981_g CU921187_s | 10.0 | 9.9 | 9.1 | 9.8 |
| BBZD_g_uc | 6.5 | 7.0 | 6.6 | 6.5 | |
| DQ415754_g_uc | 6.1 | 1.8 | 0.5 | 6.0 | |
| Porphyromonadaceae_uc | 5.3 | 5.2 | 4.7 | 5.6 | |
| Cloacamonas acidaminovorans | 4.5 | 3.3 | 4.3 | 3.2 | |
| Thermomonas carbonis | 2.1 | 1.7 | 1.6 | 2.5 | |
| Others | 65.5 | 71.1 | 73.1 | 66.4 | |
| Archaea | |||||
| Phylum | Euryarchaeota | 96.1 | 93.3 | 95.0 | 94.7 |
| Bathyarchaeota | 3.8 | 6.7 | 5.0 | 5.2 | |
| Others | 0.1 | 0.1 | 0.0 | 0.1 | |
| Genus | Methanosaeta | 44.4 | 35.7 | 30.1 | 39.9 |
| LNJC_g | 20.7 | 13.5 | 18.7 | 16.9 | |
| Methanomassiliicoccus | 15.9 | 11.4 | 10.3 | 13.9 | |
| DHVE4b_c_uc | 2.3 | 22.6 | 22.1 | 13.2 | |
| Others | 16.7 | 16.9 | 18.8 | 16.2 | |
| Species | Methanosaeta concilii | 33.1 | 27.2 | 22.0 | 30.0 |
| LNJC_g LNJC_s | 20.6 | 13.5 | 18.7 | 16.8 | |
| Methanomassiliicoccus_uc | 15.9 | 11.3 | 10.3 | 13.8 | |
| DHVE4b_c_uc | 2.3 | 22.6 | 22.1 | 13.2 | |
| Methanosaeta JN397687_s | 8.7 | 6.6 | 6.2 | 7.5 | |
| AF424768_g CU917078_s | 3.4 | 5.8 | 4.2 | 4.5 | |
| Others | 15.9 | 13.1 | 16.6 | 14.2 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piao, D.-M.; Song, Y.-C.; Oh, G.-G.; Kim, D.-H.; Bae, B.-U. Contribution of Yeast Extract, Activated Carbon, and an Electrostatic Field to Interspecies Electron Transfer for the Bioelectrochemical Conversion of Coal to Methane. Energies 2019, 12, 4051. https://doi.org/10.3390/en12214051
Piao D-M, Song Y-C, Oh G-G, Kim D-H, Bae B-U. Contribution of Yeast Extract, Activated Carbon, and an Electrostatic Field to Interspecies Electron Transfer for the Bioelectrochemical Conversion of Coal to Methane. Energies. 2019; 12(21):4051. https://doi.org/10.3390/en12214051
Chicago/Turabian StylePiao, Dong-Mei, Young-Chae Song, Gyung-Geun Oh, Dong-Hoon Kim, and Byung-Uk Bae. 2019. "Contribution of Yeast Extract, Activated Carbon, and an Electrostatic Field to Interspecies Electron Transfer for the Bioelectrochemical Conversion of Coal to Methane" Energies 12, no. 21: 4051. https://doi.org/10.3390/en12214051
APA StylePiao, D. -M., Song, Y. -C., Oh, G. -G., Kim, D. -H., & Bae, B. -U. (2019). Contribution of Yeast Extract, Activated Carbon, and an Electrostatic Field to Interspecies Electron Transfer for the Bioelectrochemical Conversion of Coal to Methane. Energies, 12(21), 4051. https://doi.org/10.3390/en12214051