Bioelectrosynthetic Conversion of CO2 Using Different Redox Mediators: Electron and Carbon Balances in a Bioelectrochemical System
Abstract
:1. Introduction
2. Materials and Methods
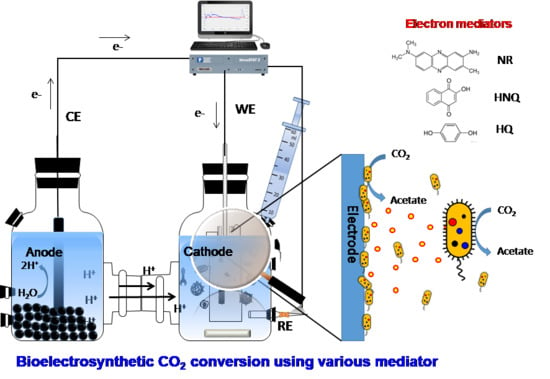
2.1. Microbial Electrosynthesis Reactor Configuration and Operation
2.2. Analysis of the Acetate Concentration and Gas Composition
2.3. Estimation of Electron Recovery and Carbon Balance and Electrochemical Analyses
2.4. Electrochemical and Microscopic Analyses of Electrosynthesis
3. Results and Discussion
3.1. Production of Acetate in MES with Different Redox Mediators
3.2. Estimation of the Electron and Carbon Recovery of Electrosynthesis
3.3. Electrochemical Characteristics of CO2 Electrosynthesis and SEM Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dudley, B. BP Statistical Review of World Energy Statistical Review of World; BP Statistical Review: London, UK, 2019; Available online: https://www.bp.com/content/dam/bp/businesssites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2019-full-report.pdf (accessed on 10 October 2019).
- Song, L.; Woo, W.T. China’s Dilemma: Economic Growth, the Environment and Climate Change; Anu E Press: Canberra, Australia, 2008. [Google Scholar]
- Zhou, J.; Zhang, F.; Meng, H.; Zhang, Y.; Li, Y. Introducing extra NADPH consumption ability significantly increases the photosynthetic efficiency and biomass production of cyanobacteria. Metab. Eng. 2016, 38, 217–227. [Google Scholar] [CrossRef]
- Gong, F.; Zhu, H.; Zhang, Y.; Li, Y. Biological carbon fixation: From natural to synthetic. J. CO2 Util. 2018, 28, 221–227. [Google Scholar] [CrossRef]
- Berg, I.A.; Kockelkorn, D.; Buckel, W.; Fuchs, G. A 3-hydroxypropionate/4-hydroxybutyrate autotrophic carbon dioxide assimilation pathway in Archaea. Science 2007, 318, 1782–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, H.; Gallenberger, M.; Jahn, U.; Eylert, E.; Berg, I.A.; Kockelkorn, D.; Eisenreich, W.; Fuchs, G. A dicarboxylate/4-hydroxybutyrate autotrophic carbon assimilation cycle in the hyperthermophilic Archaeum Ignicoccus hospitalis. Proc. Natl. Acad. Sci. USA 2008, 105, 7851–7856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, F.; Li, Y.J.S. Fixing carbon, unnaturally. Biotechnology 2016, 354, 830–831. [Google Scholar] [CrossRef] [PubMed]
- Medina-Ramos, J.; Pupillo, R.C.; Keane, T.P.; DiMeglio, J.L.; Rosenthal, J. Efficient conversion of CO2 to CO using tin and other inexpensive and easily prepared post-transition metal catalysts. J. Am. Chem. Soc. 2015, 137, 5021–5027. [Google Scholar] [CrossRef]
- Wang, H.; Matios, E.; Wang, C.; Luo, J.; Lu, X.; Hu, X.; Li, W. Rapid and scalable synthesis of cuprous halide-derived copper nano-architectures for selective electrochemical reduction of carbon dioxide. Am. Chem. Soc. 2019, 19, 3925–3932. [Google Scholar] [CrossRef] [PubMed]
- Varela, A.S.; Ranjbar Sahraie, N.; Steinberg, J.; Ju, W.; Oh, H.S.; Strasser, P. Metal-doped nitrogenated carbon as an efficient catalyst for direct CO2 electroreduction to CO and hydrocarbons. Angew. Commun. 2015, 54, 10758–10762. [Google Scholar] [CrossRef]
- Scott, K.; Yu, E.H. Microbial Electrochemical and Fuel Cells: Fundamentals and Applications; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Rabaey, K.; Rozendal, R.A. Microbial electrosynthesis—Revisiting the electrical route for microbial production. Nat. Rev. Microbiol. 2010, 8, 706. [Google Scholar] [CrossRef]
- Im, C.H.; Kim, C.; Song, Y.E.; Oh, S.-E.; Jeon, B.-H.; Kim, J.R. Electrochemically enhanced microbial CO conversion to volatile fatty acids using neutral red as an electron mediator. Chemosphere 2018, 191, 166–173. [Google Scholar] [CrossRef]
- Ter Heijne, A.; Geppert, F.; Sleutels, T.H.; Batlle-Vilanova, P.; Liu, D.; Puig, S. Mixed culture biocathodes for production of hydrogen, methane, and carboxylates. In Bioelectrosynthesis; Springer: Berlin/Heidelberg, Germany, 2017; pp. 203–229. [Google Scholar]
- Hamelers, H.V.; Ter Heijne, A.; Sleutels, T.H.; Jeremiasse, A.W.; Strik, D.P.; Buisman, C.J. New applications and performance of bioelectrochemical systems. Appl. Microbiol. Biotechnol. 2010, 85, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Ganigué, R.; Puig, S.; Batlle-Vilanova, P.; Balaguer, M.D.; Colprim, J. Microbial electrosynthesis of butyrate from carbon dioxide. Chem. Commun. 2015, 51, 3235–3238. [Google Scholar] [CrossRef]
- Bajracharya, S.; Yuliasni, R.; Vanbroekhoven, K.; Buisman, C.J.; Strik, D.P.; Pant, D. Long-term operation of microbial electrosynthesis cell reducing CO2 to multi-carbon chemicals with a mixed culture avoiding methanogenesis. Bioelectrochemistry 2017, 113, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, W.; Jiang, Y.; Su, M.; Tao, Y.; Li, D. Simultaneous microbial electrosynthesis of acetate and butyrate from carbon dioxide in bioelectrochemical systems. Chin. J. Appl. Environ. Biol. 2014, 20, 174–178. [Google Scholar]
- Kim, C.; Kim, M.Y.; Michie, I.; Jeon, B.-H.; Premier, G.C.; Park, S.; Kim, J.R. Anodic electro-fermentation of 3-hydroxypropionic acid from glycerol by recombinant Klebsiella pneumoniae L17 in a bioelectrochemical system. Biotechnol. Biofuels 2017, 10, 199. [Google Scholar] [CrossRef] [Green Version]
- Rosenbaum, M.; Aulenta, F.; Villano, M.; Angenent, L.T. Cathodes as electron donors for microbial metabolism: Which extracellular electron transfer mechanisms are involved? Bioresour. Technol. 2011, 102, 324–333. [Google Scholar] [CrossRef]
- Jiang, Y.; Su, M.; Zhang, Y.; Zhan, G.; Tao, Y.; Li, D. Bioelectrochemical systems for simultaneously production of methane and acetate from carbon dioxide at relatively high rate. Int. J. Hydrog. Energy 2013, 38, 3497–3502. [Google Scholar] [CrossRef]
- Pan, X.; Angelidaki, I.; Alvarado-Morales, M.; Liu, H.; Liu, Y.; Huang, X.; Zhu, G. Methane production from formate, acetate and H2/CO2; focusing on kinetics and microbial characterization. Bioresour. Technol. 2016, 218, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Jiang, Y.; Li, D. Production of acetate from carbon dioxide in bioelectrochemical systems based on autotrophic mixed culture. J. Microbiol. Biotechnol. 2013, 23, 1140–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arends, J.B.; Patil, S.A.; Roume, H.; Rabaey, K. Continuous long-term electricity-driven bioproduction of carboxylates and isopropanol from CO2 with a mixed microbial community. J. CO2 Util. 2017, 20, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Kracke, F.; Vassilev, I.; Krömer, J.O. Microbial electron transport and energy conservation–the foundation for optimizing bioelectrochemical systems. Front. Microbiol. 2015, 6, 575. [Google Scholar] [CrossRef] [Green Version]
- Steinbusch, K.J.; Hamelers, H.V.; Schaap, J.D.; Kampman, C.; Buisman, C.J. Bioelectrochemical ethanol production through mediated acetate reduction by mixed cultures. Environ. Sci. Technol. 2009, 44, 513–517. [Google Scholar] [CrossRef]
- Harrington, T.D.; Tran, V.N.; Mohamed, A.; Renslow, R.; Biria, S.; Orfe, L.; Call, D.R.; Beyenal, H. The mechanism of neutral red-mediated microbial electrosynthesis in Escherichia coli: Menaquinone reduction. Bioresour. Technol. 2015, 192, 689–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aulenta, F.; Di Maio, V.; Ferri, T.; Majone, M. The humic acid analogue antraquinone-2, 6-disulfonate (AQDS) serves as an electron shuttle in the electricity-driven microbial dechlorination of trichloroethene to cis-dichloroethene. Bioresour. Technol. 2010, 101, 9728–9733. [Google Scholar] [CrossRef]
- Sund, C.J.; McMasters, S.; Crittenden, S.R.; Harrell, L.E.; Sumner, J.J. Effect of electron mediators on current generation and fermentation in a microbial fuel cell. Appl. Microbiol. Biotechnol. 2007, 76, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Kim, C.; Ainala, S.K.; Bae, H.; Jeon, B.-H.; Park, S.; Kim, J.R. Metabolic shift of Klebsiella pneumoniae L17 by electrode-based electron transfer using glycerol in a microbial fuel cell. Bioelectrochemistry 2019, 125, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, M.; Elfoul-Bensaid, L.; Bernalier, A. Effect of yeast extract on growth and metabolism of H2-utilizing acetogenic bacteria from the human colon. Curr. Microbiol. 1998, 37, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Parker, V.D. The hydroquinone—Quinone redox behaviour in acetonitrile. J. Chem. Soc. D Chem. Commun. 1969, 13, 716–717. [Google Scholar] [CrossRef]
- Tschörtner, J.; Lai, B.; Krömer, J.O. Biophotovoltaics: Green power generation from sunlight and water. Front. Microbiol. 2019, 10, 866. [Google Scholar] [CrossRef] [Green Version]
- Wardman, P. Reduction potentials of one-electron couples involving free radicals in aqueous solution. J. Phys. Chem. Ref. Data 1989, 18, 1637–1755. [Google Scholar] [CrossRef] [Green Version]
- Taran, O. Electron transfer between electrically conductive minerals and quinones. Front. Chem. 2017, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Hijji, Y.M.; Barare, B.; Zhang, Y. Lawsone (2-hydroxy-1, 4-naphthoquinone) as a sensitive cyanide and acetate sensor. Sens. Actuators B Chem. 2012, 169, 106–112. [Google Scholar] [CrossRef]
- Watanabe, K.; Manefield, M.; Lee, M.; Kouzuma, A. Electron shuttles in biotechnology. Curr. Opin. Biotechnol. 2009, 20, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Miroliaei, M.R.; Samimi, A.; Mohebbi-Kalhori, D.; Khorram, M. Kinetics investigation of diversity cultures of E. coli and Shewanella sp., and their combined effect with mediator on MFC performance. J. Ind. Eng. Chem. 2015, 25, 42–50. [Google Scholar] [CrossRef]
- Penteado, E.D.; Fernandez-Marchante, C.M.; Zaiat, M.; Gonzalez, E.R.; Rodrigo, M.A. On the effects of ferricyanide as cathodic mediator on the performance of microbial fuel cells. Electrocatalysis 2017, 8, 59–66. [Google Scholar] [CrossRef]
- Im, C.H.; Song, Y.E.; Jeon, B.-H.; Kim, J.R. Biologically activated graphite fiber electrode for autotrophic acetate production from CO2 in a bioelectrochemical system. Carbon Lett. 2016, 20, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Popov, A.L.; Kim, J.R.; Dinsdale, R.M.; Esteves, S.R.; Guwy, A.J.; Premier, G.C. The effect of physico-chemically immobilized methylene blue and neutral red on the anode of microbial fuel cell. Biotechnol. Bioprocess Eng. 2012, 17, 361–370. [Google Scholar] [CrossRef]
- Rudnicka, M.; Ludynia, M.; Karcz, W. Effects of naphthazarin (DHNQ) combined with lawsone (NQ-2-OH) or 1, 4-Naphthoquinone (NQ) on the Auxin-induced growth of Zea mays L. Coleoptile Segments. Int. J. Mol. Sci. 2019, 20, 1788. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; He, N.; Zhao, Y.; Xia, D.; Wei, J.; Kang, W. Antimicrobial mechanism of hydroquinone. Appl. Biochem. Biotechnol. 2019, 189, 1291–1303. [Google Scholar] [CrossRef]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood–Ljungdahl pathway of CO2 fixation. Biochim. Biophys. Acta BBA Proteins Proteom. 2008, 1784, 1873–1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephanopoulos, G.; Aristidou, A.A.; Nielsen, J. Metabolic Engineering: Principles and Methodologies; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Rittmann, B.E.; McCarty, P.L. Environmental Biotechnology: Principles and Applications; Tata McGraw-Hill Education: New York, NY, USA, 2012. [Google Scholar]
- Seelajaroen, H.; Haberbauer, M.; Hemmelmair, C.; Aljabour, A.; Dumitru, L.M.; Hassel, A.W.; Sariciftci, N.S. Enhanced bio-electrochemical reduction of carbon dioxide by using neutral red as a redox mediator. Chembiochem 2019, 20, 1196–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Standard Reduction Potential | E° (V) vs. SHE | E° (V) vs. Ag/AgCl (3M KCl) |
|---|---|---|
| 2CO2 + 8H+ +8e− → CH3COOH + 2H2O | −0.29 | −0.5 |
| CO2 + 4H+ +4e− → CH4 + 2H2O | −0.24 | −0.45 |
| 2H+ + 2e− → H2 | −0.42 | −0.63 |
| NADH/NAD+ | −0.32 | −0.53 |
| neutral red ox/red | −0.33 | −0.54 |
| methylene blue ox/red | 0.011 | −0.2 |
| anthraquinone-2,6-disulfonate ox/red | −0.18 | −0.39 |
| methyl viologen ox/red | −0.44 | −0.65 |
| 2-hydroxy-1,4-naphthoquinone ox/red | −0.14 | −0.35 |
| hydroquinone ox/red | 0.09 | −0.12 |
| Mediator | Electrode (μg/cm2) | Suspension (μg/ mL) |
|---|---|---|
| without mediator | 1.02 ± 0.2 | 1.40 ± 0.04 × 10−2 |
| NR | 0.83 ± 0.07 | 1.50 ± 0.07 × 10−2 |
| HNQ | 0.88 ± 0.1 | 1.82 ± 0.05 × 10−2 |
| HQ | 0.67 ± 0.3 | 0.82 ± 0.1 × 10−2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Song, Y.E.; Baek, J.; Im, H.S.; Sakuntala, M.; Kim, M.; Park, C.; Min, B.; Kim, J.R. Bioelectrosynthetic Conversion of CO2 Using Different Redox Mediators: Electron and Carbon Balances in a Bioelectrochemical System. Energies 2020, 13, 2572. https://doi.org/10.3390/en13102572
Li S, Song YE, Baek J, Im HS, Sakuntala M, Kim M, Park C, Min B, Kim JR. Bioelectrosynthetic Conversion of CO2 Using Different Redox Mediators: Electron and Carbon Balances in a Bioelectrochemical System. Energies. 2020; 13(10):2572. https://doi.org/10.3390/en13102572
Chicago/Turabian StyleLi, Shuwei, Young Eun Song, Jiyun Baek, Hyeon Sung Im, Mutyala Sakuntala, Minsoo Kim, Chulhwan Park, Booki Min, and Jung Rae Kim. 2020. "Bioelectrosynthetic Conversion of CO2 Using Different Redox Mediators: Electron and Carbon Balances in a Bioelectrochemical System" Energies 13, no. 10: 2572. https://doi.org/10.3390/en13102572
APA StyleLi, S., Song, Y. E., Baek, J., Im, H. S., Sakuntala, M., Kim, M., Park, C., Min, B., & Kim, J. R. (2020). Bioelectrosynthetic Conversion of CO2 Using Different Redox Mediators: Electron and Carbon Balances in a Bioelectrochemical System. Energies, 13(10), 2572. https://doi.org/10.3390/en13102572