1. Introduction
Hermetic packaging technologies that prevent internal components from reacting with oxygen or moisture in the air are critical for numerous microscale technologies, including sensors, batteries, super-capacitors, energy harvesters and other energy systems [
1,
2,
3]. Creating suitable packaging strategies for these microscale technologies is of growing importance as the markets for these devices continue to increase. The microbattery market, for example, is predicted to grow nearly 5× between 2019 and 2025 as a result of new Internet of Things (IoT) and medical devices [
4], but current hermetic packaging technologies limit microbattery energy densities to a fraction of macroscale batteries.
One reason for the divergent energy densities of micro- and macroscale batteries is that widely used macroscale hermetic packaging technologies cannot be directly applied to microbatteries as the packaging dominates the volume and mass of the internal components [
5,
6,
7]. Battery pouch cells, for example, have a minimum hot sealing width of 3 mm, which accounts for 12% of the total cell area in a 10 × 10 cm
2 macroscale battery, and allows 88% of the cell area to be available for energy storing materials. When the same pouch cell packaging is applied to a 1 × 1 cm
2 microscale cell, the seal uses 84% of the cell area and leaves only 16% of the area for energy storing materials. If the energy storing materials had a combined energy density of 1000 Wh/L, the macroscale cell would have 880 Wh/L energy density, while the microscale cell would have only 160 Wh/L.
Due to extreme difficulties in realizing low-volume fraction microscale packaging, the field lacks available literature, and the majority of micro-energy systems studied have been demonstrated without packaging [
7,
8,
9,
10,
11,
12,
13,
14]. A microbattery by W. Lai et al. is one of the very few examples with packaging, but the bulky sealing left only 45% of the cell volume available for electrodes, which limited the full cell energy density to about 400 Wh/L [
15]. Additional prior work reduced the packaging fraction by using current collectors as part of the packaging and demonstrated the advantage of this approach [
15,
16]. In addition to requiring a low volume fraction, practical microscale hermetic packaging technologies need to be mechanically strong, chemically inert and easily assembled at scale.
Here, we presented a microscale hermetic packaging strategy that decreased the packaging volume fraction using two innovations: (1) the metal current collectors served as part of the hermetic packaging, and (2) ultra-thin strips of laser-machined hot melt tape hermetically sealed the gap between the current collectors.
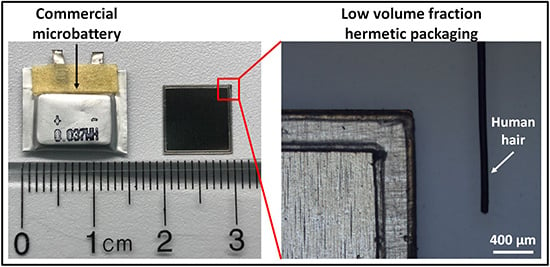
Figure 1 shows the packaging structure using a li-ion battery as an example. The volume consumed by packaging in this battery was less than 20%. This packaging volume fraction provided over a 2× improvement compared to the best lab scale microbatteries, and over a 3× improvement compared to the commercially available li-ion batteries, where packaging volume consumes as much as 70% [
17]. Furthermore, with 84% of the 1 × 1 cm
2 cell area available for active material, this strategy achieved a 5× improvement in the amount of volume available for energy storage materials compared to macroscale pouch cell sealing.
In addition to reducing the packaging volume fraction, the packaging design maintains impressive mechanical strength and stability. T-peel tests showed that the packaging structure exhibited an ultra-high adhesive strength of 43.10 N/cm, which was twice the strength of commercial pouch cells and over two times the strength of commonly used commercial metal adhesives. Additionally, the results from cyclic voltammetry and a high voltage tests showed that the packaging structure exhibited impressive electrochemical stability, with no reaction detected at up to 5 V. The presented packaging strategy offered both a significant volumetric energy density advantage and a robust, hermetic seal for micro-energy systems.
2. Materials and Methods
For the micro-device packaging, we fabricated 1 × 1 cm2 metal current collectors using laser micromachining equipment (IPG Microsystems IX-200-F, laser at 532 nm, 10 kHz; the power and pulse number varied based on different materials and thicknesses). We machined stainless steel, copper and aluminum with thicknesses between 10 μm to 50 μm. The resulting 1 × 1 cm2 metal squares represented current collectors that could be used in any microsystem, for instance as current collectors in a li-ion battery or supercapacitor on which electrode materials could be deposited.
Hot melt tape gaskets were used to create a strong hermetic seal between the metal current collectors. We made tape gaskets from 100 μm thick enhanced sulfurized polymer resin tri-layer hot melt tape from the MTI Corporation (PLIB-HMA30). Polymer resin hot melt tape was designed to seal the pouch cell cases with metal terminal tabs. Being designed for battery applications, the material has strong adhesion to metal, good electrolyte stability and low air and water permeability. An excimer laser (IPG Microsystems IX-255) with a 193 nm ultraviolet light source machined the optically transparent hot melt tape into 1 × 1 cm
2 windows with 400 μm wide boarders (
Figure 2a). The 10 μm laser resolution produced tape gaskets with only a ±5% error in edge length. By using solid gaskets and high-precision micromachining equipment, we enabled a low-volume fraction seal with significantly better dimensional precision than liquid- or gel-based adhesives.
A micro-machined hot melt tape gasket was bonded between two metal current collectors to create a 1 × 1 cm2 package for micro-energy systems. The hermetic seal was completed by hot pressing each side of the 1 × 1 cm2 package using a commercially available impulse heat sealer (FS-300, 430W, 12-inch-long, 3 mm wide filament). To achieve a strong adhesion, the tape should reach 135 °C at 0.3 MPa for 2–3 s, depending on the current collector. The heat setting was adjusted on the impulse sealer and the pressure was adjusted by the spring in the sealer. To adhere the hot melt tape onto a 50 μm thick stainless steel foil, we set the heating filament to 180 °C for 3 s which ensured that enough heat penetrated the foil to bring the adhesive to 135 °C. The heat adhered the inner hot melt tape gasket to the surrounding metal components and once cooled, created an ultra-strong solid adhesive bond.
A unique advantage to this packaging design was that the thickness could be easily adjusted for different micro-devices. To achieve higher thicknesses, we stacked multiple tape gaskets with an intermediate metal gasket between each consecutive tape layer. We obtained up to 300 μm thick gaskets, though greater thicknesses (>mm) were achievable. For the stacked layers, we fabricated metal gaskets using laser-micro-machining equipment, however, the metal gaskets had 300 μm wide windows (100 μm less than the tape gasket) so the metal would avoid contact with an inner electrode material in a battery or micro-device (
Figure 2c). Additionally, we modified the hot sealing sequence to ensure good adhesion between all the layers. We started by stacking a current collector (① in
Figure 1), a tape gasket (②) and a stainless steel gasket (③) and hot pressing the three components together (
Figure 2c). We then hot pressed on one additional tape gasket (④) with a stainless steel gasket (③’, same as ③), following a metal–polymer–metal sequence, and repeated these incremental additions until the desired thickness was achieved. The advantage to adding components in sequential steps, and never heating more than one new metal/tape/metal combination was to create easier an alignment of the packaging components and optimize the heat transfer to the tape from the impulse sealer’s one-sided heat source. After adding the intermediate stainless steel and tape layers, we completed the final step by hot pressing the remaining current collector (⑤) to the multilayer structure.
The mechanical and electrochemical properties of the micro-packaging strategy were evaluated and compared to other packaging designs. T-peel experiments tested the mechanical sealing strength between the hot melt tape and the metal current collectors. Different adhesives were used to bond two identical stainless steel strips (10 cm × 1 cm × 50 μm) at one end. Two free ends were mounted onto a mechanical tester (Instron 5564, speed at 5 mm/min), which tested the load required to peel the strips apart (
Figure 3). One sample was tested for each data point. Cyclic voltammetry scans between 0–5 V and a potentiostatic test at 5 V were performed on a dummy cell, which is a fully packed cell without electrode materials and electrolyte, to test the packaging’s electrochemical stability. The sample set up was the same as the T-peel test as shown in
Figure 3a. The bonded part was 1 × 1 cm
2 and two free ends of stainless steel strips were connected to a potentiostat (BioLogic VMP-300).
The permeability of the micro-packaging strategy was studied via the stability of lithium in a dummy cell. One piece of 0.5 × 0.5 cm2 lithium foil was sealed in a 1 × 1 cm2 dummy cell made with 50 μm thick stainless steel foils and 400 μm wide hot melt tape gasket inside an argon-filled glovebox (H2O, O2 < 1 ppm). After storing the cell at ambient condition for two months, we opened the cell and checked the lithium surface for changes.
3. Results and Discussion
The durability of stainless steel, copper and aluminum current collectors during the packaging assembly were tested. The metal current collectors provided excellent hermetic seals. Stainless steel current collectors had the highest durability and did not fracture during assembly even at a low 10 μm thickness. In comparison, thin copper and aluminum foils were more susceptible to coiling and tearing. Without electrode materials to mechanically reinforce the current collectors, copper and aluminum required greater than 50 μm thicknesses to avoid failure. Lower thicknesses were possible when combined with electrode materials. With the aim of minimizing the material volume, we determined 10 μm stainless steel foils were optimal current collectors for our 1 × 1 cm2 packaging.
The T-peel tests showed that the adhesion between the tape gaskets and current collectors exhibited an ultra-high strength of 43.10 N/cm.
Table 1 compares the strength of this packing structure with other materials commonly used in electronic device packaging. As illustrated, this work presented a packaging strategy with twice the peel strength of commercial pouch cell cases and significantly higher than the popular industrial adhesives.
We assessed the electrochemical stability of the packaging structure through cyclic voltammetry and constant high voltage tests (
Figure 4a,b). We used a dummy cell, in which there was no active material on the metal current collectors, to test the electrochemical response of the packaging components only. The experiment tested the stability between 0 to 5 V. The detected currents were in the range of equipment background and noise, which also proved the insulating capability of the sealing structure. The results from the CV and high-voltage test showed that the packaging structure exhibited impressive electrochemical stability, with no reaction detected even at 5 V.
The lithium stability test also demonstrated the excellent hermetic property of the packaging structure.
Figure 4c shows the lithium sealed inside the dummy cell and stored in ambient condition for two months. The lithium kept its original shinny metallic surface which indicated that no detectable water or oxygen leaked through the 400 μm wide hot melt tape gasket.
We developed a low volume fraction micro-packaging strategy designed to increase the volumetric and gravimetric energy density of micro-energy storage technologies. This approach improved upon standard MEMS packaging solutions where the total area, but not necessarily weight and volume, are important [
15]. In addition, our approach can be applied with moderate temperatures and is compatible with organic and corrosive solvents. Many MEMS-packaging solutions, in contrast, use glass and silicon bonding which require very high temperatures that adversely affect the critical microbattery components, like the separator, and can cause phase changes in the active electrodes [
18]. Many microfluidic device-packaging strategies have also been designed to contain fluids, but common materials like polydimethylsiloxane (PDMS) and epoxies are not compatible with the organic solvent and corrosive salts in energy technologies [
19]. Our packaging strategy overcomes the material and processing limitations of prior packaging solutions [
20].