1. Introduction
Internal combustion engines (ICEs) are important power sources and are responsible for around 60% of all oil produced [
1]. However, the brake thermal efficiency and fuel economy of current engines are still quite low. One of the reasons that limit further improvement is that part of the heat energy from fuel combustion is wasted to the ambient through multiple media such as exhaust gas, jacket water, turbo-charge coolant, exhaust gas recirculation coolant, etc. [
2]. Hence, great potential in engine waste heat recovery has been put forward and widely concerned if the waste heat can be transformed into mechanical or electricity power. The Rankin cycle (RC) is supposed to be a promising bottoming cycle technology when engine waste heat recovery is set as the topping cycle [
3].
Many researches about RCs are devoted in biomass [
4], solar power plants [
5], geothermal [
6], industrial waste heat [
7], combined heat and power generation [
8], etc. As for the RCs applied to the engine waste heat recovery, the first study proposed may be traced to the 1970s when Patel et al. [
9] compounded the truck diesel engine with a bottoming cycle of the subcritical organic Rankine cycle (ORC) system. A great improvement of 15% in fuel economy assumption was achieved by means of the aforementioned conceptual design. Since then, the subcritical ORC technologies were shown as exceptional and effective features for the characteristics of small volume, light weight, low cost, no invasive property compared with turbo-compounding, energy efficiency improvement, and environmental protection [
10] and attracted a widespread attention. When different organic working fluids are adopted, the performances of ORCs vary. Rad et al. [
11] selected potential working fluids for ORCs in industrial waste heat recovery under various heat source temperatures. It could be deduced that when the critical temperature of working fluids such as water was near the heat source temperature, the highest energy efficiency could be achieved mainly due to a lower temperature difference between the heat source and working fluid. Mikielewicz et al. [
12] conducted thermodynamic performances of an ORC system with various working fluids integrated with a 900 MWe power plant. They recommended ethanol, the best-performing working fluid with the highest exergetic efficiency of 78.90%. Akbari et al. [
13] focused on a newly designed systematic selection approach of the optimal working fluid in order to minimize the total thermal conductance of the system. They found that c2butene needs the smallest heat exchanger surface area compared to R600a, R601a, and R245fa. Nevertheless, the working fluids used in the conventional subcritical ORCs with their critical temperatures less than the temperature of the heat source will present poor system performances. The reason can be explained by large irreversibility between the working fluid and heat source caused by the isothermal phase change of the working fluid [
14].
Various investigations have been performed in the literature for minimizing the aforementioned large irreversibility, as well as decreasing the exergy destruction of the system, which results in a higher performance. The utilization of zeotropic mixtures serves as a potential solution. It is found that a temperature glide occurs in the phase change procedure for zeotropic mixtures, which contributes to a better temperature-matching performance and the enhancement of net power and exergy efficiency. Zhi et al. [
15] studied ORCs with R600a/R601a and R134a/R245fa mixtures as working fluids in engine waste heat recovery. The maximum increase of 19.78% in power output (97.95 kW) could be obtained with the mixtures of R600a/R601a (0.3/0.7) and R134a/R245fa (0.4/0.6) compared with pure working fluids. Andreasen et al. [
16] conducted four different methods to make comparisons of selected 30 different pure fluids and zeotropic mixtures on heat exchanger performances and illustrated the benefits of using zeotropic mixtures. Interesting results showed that net power outputs of zeotropic mixtures could be improved by up to 13.6% compared to the best pure working fluids when the same minimum pinch point temperature differences for all fluids were taken into consideration. Similar research on zeotropic mixtures by Zühlsdorf et al. [
17] in heat pumps revealed that a simultaneous improvement of thermodynamic performance and levelized specific cost could be achieved when the mixture of 30% propylene and 70% R-1234ze(Z) was adopted in the case study since the mixture showed a better temperature match with the temperature profile of the sink and source during their heat transfer. Chys et al. [
18] investigated zeotropic mixtures in a geothermal power plant, and they found a great increase in energy efficiency of approximately 16% and 6% under 250 and 150°C heat source temperatures when the zeotropic mixture was compared with pure fluid. Lecompte et al. [
19] conducted exergetic analysis of an ORC system considering various composition mixtures. The maximum exergetic efficiency of 32.05% could be achieved when the isobutane–isopentane with a concentration of 0.81/0.19 was selected as the optimal composition. The results were mainly ascribed to decreasing the exergy destruction in the condenser.
The transcritical Rankine cycle (TRC), another potential solution to the temperature match problem, is introduced to improve the temperature match between the working fluid and heat source. Hence, the comparison between the subcritical ORC and TRC is carried out. Hsieh et al. [
20] conducted an experimental comparison of five cases when the working fluid R218 was pressurized to the subcritical or supercritical states. Apparent increases in the mass flow rate and heat absorption rate were found when the TRCs were achieved. Tian et al. [
21] evaluated the performance of a TRC system considering the thermophysical properties of the working fluid and presented a selection principle of working fluids under different heat source temperatures. Shu et al. [
22] conducted a theoretic analysis on a dual-loop TRC system in an engine waste heat recovery to overcome the large temperature differences between the high-temperature exhaust gas and low-temperature engine jacket water. Six candidate working fluids were compared and analyzed. Li et al. [
23] proposed a novel configuration coupling the supercritical and subcritical heat absorption processes to increase the heat-power conversion efficiency and to improve the adaptability to heat sources. Meng et al. [
24] conducted a thermoeconomic performance comparison between the TRC and ORC in a low-temperature heat source utilization. The authors found that the net power output could be improved by 128.5% at maximum for the TRC and considered it a great potential in low-temperature heat source recovery due to its good environmental properties.
In addition to cycle comparison and configuration modification, a key design factor of the TRCs is the working fluid selection. Recently, a growing number of scholars have devoted their efforts to selecting the best working fluid from numerous candidates in the specific application area considering various optimization objectives. On one hand, when the operation stability and compatibility with materials of different working fluids are investigated as criteria, several indicators are included, mainly considering the working fluid properties. Liu et al. [
25] firstly proposed concepts of dry, wet, and isentropic working fluids classified by the positive, negative, and infinite slope of the T-s curve, respectively. Further assertion was given by Hung et al. [
26] that isentropic or dry fluids were suggested to avoid liquid droplet impingement in the turbine blades during the expansion. One solution to the use of wet working fluids was given by Desai et al. [
27] to ensure the superheated state at the turbine inlet. On the other hand, when the thermodynamic performances of different working fluids are studied, a brief review is also given below. The critical temperature of different working fluids showed great impacts on system thermodynamic performances. Heberle et al. [
28] compared the second law of efficiency of various fluids and recommended that the working fluids with high critical temperatures like isopentane were suitable for the ORCs. Similar results could be found when the boiling temperatures of fluids were compared. Actually, the relations between the critical temperature and boiling temperature were given by Joback [
29] that the boiling temperature became higher if the critical temperature became higher for the fluids in the same fluid family. Besides the critical temperature, the ratio of latent heat to sensible heat is commonly used and modified to evaluate exergetic performances of working fluids. The specific heat of a working fluid is an important property, since the direct impact is caused by different values of specific heats on the pressurization and expansion processes. Maizza and Papadopoulos et al. [
30,
31] believed that the lower specific heat would decrease the work consumed by the pump and increase the power output indirectly. Furthermore, a higher specific heat was better-suited to obtain large expansion work. Stijepovic et al. [
32] observed that working fluids with a low ratio of latent heat to sensible heat, which would be inclined to extract more heat from the heat source, showed better performances regarding exergy efficiency. A combination of the defined Jakob number and the average temperature between the evaporation temperature and condensation temperature was deduced by Su et al. [
33]. They recommended it as more favorable for system thermal efficiency when a smaller Jakob number was achieved. The molecular complexity and molecular weight of different working fluids also has an impact on the system thermodynamic performance, especially on the expansion process [
34]. As for the environmental aspects, the main concerns included the ozone depletion potential (ODP), global warming potential (GWP), and the atmospheric lifetime (ALT).
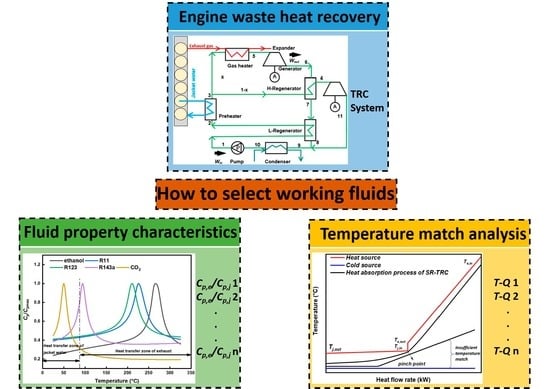
After a concise literature review about different performances of working fluids in TRCs and fluid selection instructions, it is concluded that the way to improve the temperature match between working fluids and thermal sources such as zeotropic mixtures and cycle modification shows a great impact on systems’ performances. In addition, the reason why different thermodynamic performances of different working fluids are performed can be derived from the multiple properties of working fluids. Nevertheless, a current limitation of the reviewed working fluid selection is that a sufficient selection condition of what kind of properties the best working fluid possesses is proposed only after the comparison of their thermodynamic or economic performances. It seems to show a lack of active fluid selection instruction from the perspective of what kind of properties better-working fluids should possess. Additionally, the thermodynamic and economic performances of selected working fluids are served as a validation of the active fluid selection instruction. Hence, based on the temperature match method, which has been proved to be an effective and efficient way to achieve configuration modification, an optimal TRC layout is proposed to accomplish the active working fluid selection. The illustration and validation of the approach will allow the development of selecting working fluids actively from another dimension. Hence, the aim of the study is twofold: (i) set up a temperature match method-based working fluid selection instruction to obtain better working fluid properties with better temperature match performances between the bottoming system and multiple engine heat sources, as well as the cold source, and (ii) conduct a thermodynamic and economic analysis to validate the proposed method and optimize important operation parameters.
3. Mathematical Modeling
The study is performed by means of a thermodynamic and economic analysis focusing on the energetic, exergetic, and economic performances. Several reasonable assumptions below are defined to simplify the computation process [
37,
38,
39]: (1) each component and operating point is under equilibrium and in a steady-state condition; (2) heat losses and pressure losses are neglected in the pipes and in all components; (3) the isentropic efficiencies of the expander, pump, and generator are assumed to be 0.7, 0.8, and 0.9, respectively; (4) the changes in the kinetic and potential energy of the fluids can be neglected; (5) the pinch point temperature differences (
Tpp) in the gas heater, preheater, and regenerator are set to be 30 °C, 5 °C, and 15 °C; and (6) temperatures of the condensation process and the ambient are set at 25 °C to guarantee that CO
2 can be condensed stably during the phase change process. The properties of the working fluid are derived from the REFPROP-NIST [
40].
3.1. Thermodynamic Analysis
Thermodynamic modeling of the combined system can be constituted by the energetic and exergetic terms. Based on the first law of thermodynamics, the energy balance equations of the system components are listed in the second column of
Table 4. An exergy analysis based on the second law of thermodynamics is necessary to judge the irreversibility of each component during the investigation of the cycles. The exergy loss equations of the system components are listed in the third column of
Table 4. To make full use of one heat source at least, the mass flow rate (
) of the working fluid is dependent on the minimum value of
and
, which are calculated by:
In order to ensure the stable operation of the heat exchanger, the outlet temperature of the exhaust gas from the gas heater should not be lower than the acid dew point temperature (120 °C) to prevent the corrosion of the heat exchanger [
39]. In addition, the outlet temperature of the jacket water after the preheater should be higher than the returned temperature to avoid an unnecessary impact on the engine operation. Based on the aforementioned conditions, the heat recovery efficiency and system total efficiency are defined as:
3.2. Economic Analysis
Considering the characteristics of the heat exchanger-based split cycles, the heat transfer areas of the heat exchangers are calculated by:
where
K is the total heat transfer coefficient of the heat exchanger, and Δ
t is the log mean temperature difference (LMTD) between the hot side and the cold side.
To determine the unit cost of the streams in the conversion system, the capital cost according to the economic situation in the year 2014 is expressed as:
where
CEPCI2001 = 382 and
CEPCI2014 = 586.77 (CEPCI means chemical engineering plant cost index).
The purchased cost
Cp and the cost factor
Fbm are two main parameters that affect the base investment cost of each component. Here, the
Cp is calculated by the capacity of the components, and the relevant coefficient
K, and the cost factors, including direct project expenses, contingency and contractor fees, indirect project expenses, and the auxiliary facilities, are taken into consideration by
Fbm. The relevant investment models and coefficients according to [
41] are listed in
Table 5. In addition, the capital recovery (CRF) is estimated as:
where
i is the interest rate, and the value is set to be 5%;
time is the economic lifetime, and its value is set to 15 years. The annuity of the investment
Ank can be expressed as:
The electricity production cost (EPC) can be calculated by the equation:
where
fk is the operation, maintenance, and insurance cost factor, and its value is 1.65%;
h is the operation hours of a year, and its value is set to be 2190 h considering the practical operation.
The aforementioned calculations were conducted through MATLAB. To help readers get a good grasp of the calculation and optimization procedure, the flow chart is given in
Figure 2.
3.3. Model Validation
The model of the transcritical Rankine cycle system with a preheater and a regenerator was verified with the same operating parameters as in reference [
42], and the comparison results are listed in
Table 6 based on the thermodynamic analysis as follows. A 25-MW gas turbine was used as the heat source under a condensing temperature
Tcond of 20 °C and a expander inlet temperature
Texpander of 391 °C. The
RD (relative deviation) is described as:
The results calculated in this paper are in a good agreement with those of the reference. Therefore, the models in this paper are sufficiently accurate for our investigation.
3.4. Working Fluid Screening
Working fluid selection is very important, since it directly influences performances such as net power output, thermal efficiency, and heat transfer area, etc. The choice of the working fluid is a complex problem, because it implies the selection of the appropriate working fluid among a large number of candidates. A concise literature review has been carried out in the Introduction section. In this study, 18 different and commonly used working fluids are listed in
Table 7, and the primary selection was conducted by means of their pyrolytic decomposition temperatures. Considering the high temperature of the exhaust gas (469.4 °C), the candidates, which had higher pyrolytic decomposition temperatures than 300 °C, were selected and analyzed to avoid the decomposition problem. Hence, the final working fluids selected included CO
2, R143a, R123, R11, and ethanol.
It is worth noting that the mentioned working fluids, such as R11 (ODP = 1, GWP = 1), R143a (ODP = 0, GWP = 3800), and R22 (ODP = 0.055, GWP = 1700), may cause severe environmental impacts, including depletion of the ozone layer and global warming, and have been banned or limited in use in future equipment by the Montreal Protocol on Substances that Deplete the Ozone Layer and Kyoto Protocol. Nevertheless, in order to give a clear explanation of the proposed method of active fluid selection, the aforementioned candidates are only used for the validation of the fluid selection method from the temperature match standpoint, despite some of them being restricted from the perspective of environmental protection.