Determination of Various Parameters during Thermal and Biological Pretreatment of Waste Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock Preparation and Characterization
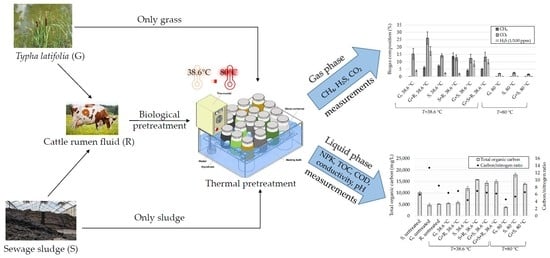
2.2. Experimental Setup
2.3. Chemical Analyses
3. Results and Discussion
3.1. Feedstock Characterization Results
3.2. Results of the Pretreatment Tests
3.2.1. Results of N, P and K Analysis
3.2.2. TOC Values and C/N Ratio
3.2.3. Chemical Oxygen Demand (Soluble COD) Measurements
3.2.4. pH and Conductivity
3.2.5. Gas Phase Composition
4. Conclusions and Future Research
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| COD | Chemical oxygen demand |
| C/N | Carbon/Nitrogen ratio |
| DM | Dry matter |
| G | Grass Typha latifolia |
| ICP-OES | Inductively coupled plasma optical emission spectrometry |
| NPK | Nitrogen (N), phosphorus (P2O5) and potassium (K2O) |
| PE | Population Equivalent |
| POP | Persistent organic pollutants |
| R | Rumen fluid |
| S | Sewage sludge |
| TC | Total carbon |
| TN | Total nitrogen |
| TOC | Total organic carbon |
| TP | Total phosphorus |
| TS | Total solids |
| VFA | Volatile fatty acid |
References
- Čuček, L.; Klemeš, J.J.; Varbanov, P.S.; Kravanja, Z. Significance of environmental footprints for evaluating sustainability and security of development. Clean Technol. Environ. Policy 2015, 17, 2125–2141. [Google Scholar] [CrossRef]
- Bezama, A.; Agamuthu, P. Addressing the Big Issues in Waste Management; SAGE: London, UK, 2019. [Google Scholar]
- Ferronato, N.; Torretta, V. Waste Mismanagement in Developing Countries: A Review of Global Issues. Int. J. Environ. Res. Public Health 2019, 16, 1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Commission. Waste. Available online: ec.europa.eu/environment/waste/index.htm (accessed on 28 March 2020).
- Balasubramanian, S.; Tyagi, R.D. 2—Value-Added Bio-products From Sewage Sludge. In Current Developments in Biotechnology and Bioengineering; Wong, J.W.C., Tyagi, R.D., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–42. [Google Scholar]
- Alibardi, L.; Cossu, R. Composition variability of the organic fraction of municipal solid waste and effects on hydrogen and methane production potentials. Waste Manag. 2015, 36, 147–155. [Google Scholar] [CrossRef] [PubMed]
- European Parliament and the Council of the European Union. Directive (EU) 2018/850 of the European Parliament and of the Council of 30 May 2018 Amending Directive 1999/31/EC on the Landfill of Waste. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018L0850&from=EN (accessed on 3 May 2020).
- Behera, S.; Arora, R.; Nandhagopal, N.; Kumar, S. Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2014, 36, 91–106. [Google Scholar] [CrossRef]
- Anawar, H.M.; Strezov, V. Technical and Economic Assessment of Biogas and Liquid Energy Systems from Sewage Sludge and Industrial Waste: Lifecycle Assessment and Sustainability. In Renewable Energy Systems from Biomass; CRC Press: Boca Raton, FL, USA, 2018; pp. 57–73. [Google Scholar]
- Fatma, S.; Hameed, A.; Noman, M.; Ahmed, T.; Shahid, M.; Tariq, M.; Sohail, I.; Tabassum, R. Lignocellulosic biomass: A sustainable bioenergy source for the future. Protein Pept. Lett. 2018, 25, 148–163. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef] [Green Version]
- Seidl, P.R.; Goulart, A.K. Pretreatment processes for lignocellulosic biomass conversion to biofuels and bioproducts. Curr. Opin. Green Sustain. Chem. 2016, 2, 48–53. [Google Scholar] [CrossRef]
- Kumar, A.K.; Parikh, B.S. Cellulose-degrading enzymes from Aspergillus terreus D34 and enzymatic saccharification of mild-alkali and dilute-acid pretreated lignocellulosic biomass residues. Bioresour. Bioprocess. 2015, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Zhang, P.; Ye, J.; Wu, Y.; Fang, W.; Gou, X.; Zeng, G. Improvement of methane production from rice straw with rumen fluid pretreatment: A feasibility study. Int. Biodeterior. Biodegrad. 2016, 113, 9–16. [Google Scholar] [CrossRef]
- Rouches, E.; Herpoël-Gimbert, I.; Steyer, J.P.; Carrere, H. Improvement of anaerobic degradation by white-rot fungi pretreatment of lignocellulosic biomass: A review. Renew. Sustain. Energy Rev. 2016, 59, 179–198. [Google Scholar] [CrossRef]
- Mahmud, N.; Rosentrater, K.A. Life-Cycle Assessment (LCA) of Different Pretreatment and Product Separation Technologies for Butanol Bioprocessing from Oil Palm Frond. Energies 2020, 13, 155. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Karimi, K.; Taherzadeh, M.J. A critical review on analysis in pretreatment of lignocelluloses: Degree of polymerization, adsorption/desorption, and accessibility. Bioresour. Technol. 2016, 203, 348–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SIST EN 16168:2013. Sludge, Treated Biowaste and Soil—Determination of Total Nitrogen Using Dry Combustion Method; Slovenian Institute for Standardization (SIST): Ljubljana, Slovenia, 2013. [Google Scholar]
- SIST EN ISO 11885:2009. Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) (ISO 11885:2007); Slovenian Institute for Standardization (SIST): Ljubljana, Slovenia, 2009. [Google Scholar]
- SIST EN 13137:2002. Characterization of Waste—Determination of Total Organic Carbon (TOC) in Waste, Sludges and Sediments; Slovenian Institute for Standardization (SIST): Ljubljana, Slovenia, 2002. [Google Scholar]
- SIST EN 14346:2007. Characterization of Waste—Alculation of Dry Matter by Determination of Dry Residue or Water Content; Slovenian Institute for Standardization (SIST): Ljubljana, Slovenia, 2007. [Google Scholar]
- EN 16170:2016. Sludge, Treated Biowaste and Soil—Determination of Elements Using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES); European Committee for Standardization: Brussels, Belgium, 2016. [Google Scholar]
- Turbill, C.; Ruf, T.; Mang, T.; Arnold, W. Regulation of heart rate and rumen temperature in red deer: Effects of season and food intake. J. Exp. Biol. 2011, 214, 963–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fijalkowski, K.; Rorat, A.; Grobelak, A.; Kacprzak, M.J. The presence of contaminations in sewage sludge—The current situation. J. Environ. Manag. 2017, 203, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Bedoić, R.; Čuček, L.; Ćosić, B.; Krajnc, D.; Smoljanić, G.; Kravanja, Z.; Ljubas, D.; Pukšec, T.; Duić, N. Green biomass to biogas—A study on anaerobic digestion of residue grass. J. Clean. Prod. 2019, 213, 700–709. [Google Scholar] [CrossRef]
- Marliah, A.; Anhar, A.; Hayati, E. Combine organic and inorganic fertilizer increases yield of cucumber (Cucumissativus L.). IOP Conf. Ser. Earth Environ. Sci. 2020, 425, 012075. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Nguyen, A.Q.; Johir, M.A.H.; Guo, W.; Ngo, H.H.; Chaves, A.V.; Nghiem, L.D. Application of rumen and anaerobic sludge microbes for bio harvesting from lignocellulosic biomass. Chemosphere 2019, 228, 702–708. [Google Scholar] [CrossRef]
- Zou, J.; Li, Y. Anaerobic fermentation combined with low-temperature thermal pretreatment for phosphorus-accumulating granular sludge: Release of carbon source and phosphorus as well as hydrogen production potential. Bioresour. Technol. 2016, 218, 18–26. [Google Scholar] [CrossRef]
- Kuroda, A.; Takiguchi, N.; Gotanda, T.; Nomura, K.; Kato, J.; Ikeda, T.; Ohtake, H. A simple method to release polyphosphate from activated sludgefor phosphorus reuse and recycling. Biotechnol. Bioeng. 2002, 78, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Hudcová, H.; Vymazal, J.; Rozkošný, M. Present restrictions of sewage sludge application in agriculture within the European Union. Soil Water Res. 2019, 14, 104–120. [Google Scholar] [CrossRef]
- Boutchich, G.E.K.; Tahiri, S.; Mahi, M.; Gallart-Mateu, D.; de la Guardia, M.; Aarfane, A.; Lhadi, E.; El Krati, M. Characterization of activated sludge from domestic sewage treatment plants and their management using composting and co-composting in aerobic silos. J. Mater. Env.. Sci. 2015, 6, 2206–2220. [Google Scholar]
- Liu, L.; Xu, X.; Cao, Y.; Cai, C.; Cui, H.; Yao, J. Nitrate decreases methane production also by increasing methane oxidation through stimulating NC10 population in ruminal culture. AMB Express 2017, 7, 76. [Google Scholar] [CrossRef]
- Wang, X.; Yang, R.; Guo, Y.; Zhang, Z.; Kao, C.M.; Chen, S. Investigation of COD and COD/N ratio for the dominance of anammox pathway for nitrogen removal via isotope labelling technique and the relevant bacteria. J. Hazard. Mater. 2019, 366, 606–614. [Google Scholar] [CrossRef]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Abecia, L.; Angarita, E.; Aravena, P.; Nora Arenas, G.; Ariza, C.; et al. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci. Rep. 2015, 5, 14567. [Google Scholar] [CrossRef]
- Campanaro, S.; Treu, L.; Cattani, M.; Kougias, P.G.; Vendramin, V.; Schiavon, S.; Tagliapietra, F.; Giacomini, A.; Corich, V. In vitro fermentation of key dietary compounds with rumen fluid: A genome-centric perspective. Sci. Total Environ. 2017, 584–585, 683–691. [Google Scholar] [CrossRef]
- Xing, B.-S.; Han, Y.; Wang, X.C.; Ma, J.; Cao, S.; Li, Q.; Wen, J.; Yuan, H. Cow manure as additive to a DMBR for stable and high-rate digestion of food waste: Performance and microbial community. Water Res. 2020, 168, 115099. [Google Scholar] [CrossRef]
- Risberg, K.; Sun, L.; Levén, L.; Horn, S.J.; Schnürer, A. Biogas production from wheat straw and manure–impact of pretreatment and process operating parameters. Bioresour. Technol. 2013, 149, 232–237. [Google Scholar] [CrossRef]
- Baba, Y.; Tada, C.; Fukuda, Y.; Nakai, Y. Improvement of methane production from waste paper by pretreatment with rumen fluid. Bioresour. Technol. 2013, 128, 94–99. [Google Scholar] [CrossRef]
- Karthikeyan, O.P.; Visvanathan, C. Bio-energy recovery from high-solid organic substrates by dry anaerobic bio-conversion processes: A review. Rev. Environ. Sci. Bio/Technol. 2013, 12, 257–284. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Raghavan, V.; Palacios, J.H.; Grenier, M.; Zegan, D. The Production of Engineered Biochars in a Vertical Auger Pyrolysis Reactor for Carbon Sequestration. Energies 2017, 10, 288. [Google Scholar] [CrossRef] [Green Version]
- Venkatesh, M. Appraisal of the carbon to nitrogen (C/N) ratio in the bed sediment of the Betwa River, Peninsular India. Int. J. Sediment Res. 2020, 35, 69–78. [Google Scholar] [CrossRef]
- Yang, S.; Wang, W.; Wei, H.; Van Wychen, S.; Pienkos, P.T.; Zhang, M.; Himmel, M.E. Comparison of Nitrogen Depletion and Repletion on Lipid Production in Yeast and Fungal Species. Energies 2016, 9, 685. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Liu, J.; Yuan, X.; Wang, X.; Zhu, W.; Yang, F.; Cui, Z. Effect of dairy manure to switchgrass co-digestion ratio on methane production and the bacterial community in batch anaerobic digestion. Appl. Energy 2015, 151, 249–257. [Google Scholar] [CrossRef]
- Zhu, N. Effect of low initial C/N ratio on aerobic composting of swine manure with rice straw. Bioresour. Technol. 2007, 98, 9–13. [Google Scholar] [CrossRef]
- Żukowska, G.; Mazurkiewicz, J.; Myszura, M.; Czekała, W. Heat Energy and Gas Emissions during Composting of Sewage Sludge. Energies 2019, 12, 4782. [Google Scholar] [CrossRef] [Green Version]
- Neshat, S.A.; Mohammadi, M.; Najafpour, G.D.; Lahijani, P. Anaerobic co-digestion of animal manures and lignocellulosic residues as a potent approach for sustainable biogas production. Renew. Sustain. Energy Rev. 2017, 79, 308–322. [Google Scholar] [CrossRef]
- Zver, A.; Bernik, R.; Mihelič, R. Effect of Pressure on the Removal of NH3 from Hydrolyzed and Pre-Fermented Slaughterhouse Waste for Better Biomethanization. Energies 2019, 12, 1868. [Google Scholar] [CrossRef] [Green Version]
- Steffen, R.; Szolar, O.; Braun, R. Feedstocks for Anaerobic Digestion; Institute of Agrobiotechnology Tulin, University of Agricultural Sciences: Vienna, Austria, 1998. [Google Scholar]
- Čater, M.; Zorec, M.; Logar, R.M. Methods for improving anaerobic lignocellulosic substrates degradation for enhanced biogas production. Springer Sci. Rev. 2014, 2, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.-H.; Yu, H.-Q. Anaerobic digestion of cattail by rumen cultures. Waste Manag. 2006, 26, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Ariunbaatar, J.; Panico, A.; Yeh, D.H.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Enhanced mesophilic anaerobic digestion of food waste by thermal pretreatment: Substrate versus digestate heating. Waste Manag. 2015, 46, 176–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dölle, K.; Wagmüller, F. Determination of the Chemical Oxygen Demand (COD) of Hydrothermal Pretreated Hay Samples. Curr. J. Appl. Sci. Technol. 2015, 8, 356–360. [Google Scholar] [CrossRef]
- Zhang, J.; Li, D.; Wang, C.; Ding, Q. An Intelligent Four-Electrode Conductivity Sensor for Aquaculture. In Proceedings of the Computer and Computing Technologies in Agriculture VI, Zhangjiajie, China, 19–21 October 2012; pp. 398–407. [Google Scholar]
- Budiyono, B.; Widiasa, I.N.; Johari, S.; Sunarso, S. Increasing biogas production rate from cattle manure using rumen fluid as inoculums. Int. J. Sci. Eng. 2014, 6, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Xing, B.-S.; Han, Y.; Wang, X.C.; Wen, J.; Cao, S.; Zhang, K.; Li, Q.; Yuan, H. Persistent action of cow rumen microorganisms in enhancing biodegradation of wheat straw by rumen fermentation. Sci. Total Environ. 2020, 715, 136529. [Google Scholar] [CrossRef] [PubMed]
- Lange, L.; Pilgaard, B.; Herbst, F.-A.; Busk, P.K.; Gleason, F.; Pedersen, A.G. Origin of fungal biomass degrading enzymes: Evolution, diversity and function of enzymes of early lineage fungi. Fungal Biol. Rev. 2019, 33, 82–97. [Google Scholar] [CrossRef]
- Bermúdez-Penabad, N.; Kennes, C.; Veiga, M.C. Anaerobic digestion of tuna waste for the production of volatile fatty acids. Waste Manag. 2017, 68, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Carotenuto, C.; Guarino, G.; Morrone, B.; Minale, M. Temperature and pH effect on methane production from buffalo manure anaerobic digestion. Int. J. Heat Technol. 2016, 34, S425–S429. [Google Scholar] [CrossRef]
- Wang, W.C.; Sastry, S.K. Changes in electrical conductivity of selected vegetables during multiple thermal treatments. J. Food Process Eng. 1997, 20, 499–516. [Google Scholar] [CrossRef]
- Le Ruyet, P.; Tucker, W. Ruminal buffers: Temporal effects on buffering capacity and pH of ruminal fluid from cows fed a high concentrate diet. J. Dairy Sci. 1992, 75, 1069–1077. [Google Scholar] [CrossRef]
- Qiao, W.; Yan, X.; Ye, J.; Sun, Y.; Wang, W.; Zhang, Z. Evaluation of biogas production from different biomass wastes with/without hydrothermal pretreatment. Renew. Energy 2011, 36, 3313–3318. [Google Scholar] [CrossRef]
- Long, Y.; Fang, Y.; Shen, D.; Feng, H.; Chen, T. Hydrogen sulfide (H2S) emission control by aerobic sulfate reduction in landfill. Sci. Rep. 2016, 6, 38103. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Idler, C.; Ammon, C.; Herrmann, C.; Amon, T. Inactivation of ESBL-/AmpC-producing Escherichia coli during mesophilic and thermophilic anaerobic digestion of chicken manure. Waste Manag. 2019, 84, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ramírez, J.E.; Pastor, L.; Martí, N.; Claros, J.; Doñate, S.; Bouzas, A. Analysis of uncontrolled phosphorus precipitation in anaerobic digesters under thermophilic and mesophilic conditions. Environ. Technol. 2019, 1–10. [Google Scholar] [CrossRef]
- De Vrieze, J.; Hennebel, T.; Boon, N.; Verstraete, W. Methanosarcina: The rediscovered methanogen for heavy duty biomethanation. Bioresour. Technol. 2012, 112, 1–9. [Google Scholar] [CrossRef]





| Reaction Mixture | Rumen Fluid (mL) | Typha latifolia Grass (g) | Sewage Sludge (g) | Water (g) |
|---|---|---|---|---|
| G (grass) | / | 405.40 | / | 94.60 |
| S (sludge) | / | / | 161.27 | 338.73 |
| G + S | / | 202.70 | 80.65 | 216.65 |
| G + R | 50 | 405.40 | / | 94.60 |
| S + R | 50 | / | 161.27 | 338.70 |
| G + S + R | 50 | 202.70 | 80.65 | 216.65 |
| Parameter | Sewage Sludge (S) | Typha latifolia Grass (G) | Rumen Fluid (R) |
|---|---|---|---|
| Total dry solids (TS, %) | 18.60 | 7.40 | 2.34 |
| Water content (%) | 81.40 | 92.60 | 97.66 |
| TN (% TS) | 7.85 | 3.46 | 3.70 |
| TP (% TS) | 0.92 | 0.32 | 0.07 |
| TC (% TS) | 45.61 | 46.62 | 31.22 |
| Cd (mg/kg DM) | 1.01 | 1.03 | / |
| Cu (mg/kg DM) | 172.82 | 6.42 | / |
| Cr (mg/kg DM) | 46.36 | 1.39 | / |
| Zn (mg/kg DM) | 739.99 | 27.03 | / |
| Ni (mg/kg DM) | 24.55 | 1.72 | / |
| Pb (mg/kg DM) | 27.06 | 1.91 | / |
| K (mg/kg DM) | 8,211.80 | 34,398.43 | / |
| Sample | K2O (mg/L) | P2O5 (mg/L) |
|---|---|---|
| G, untreated | 1486 ± 89 | 476 ± 8 |
| S, untreated | 1120 ± 43 | 3554 ± 95 |
| R, untreated | 731 ± 19 | 1078 ± 21 |
| G, 38.6 °C | 5823 ± 47 | 679 ± 10 |
| G + R, 38.6 °C | 3092 ± 151 | 541 ± 26 |
| S, 38.6 °C | 2015 ± 113 | 4725 ± 77 |
| S + R, 38.6 °C | 3193 ± 95 | 3974 ± 49 |
| G + S, 38.6 °C | 11,747 ± 124 | 4146 ± 128 |
| G + S + R, 38.6 °C | 9940 ± 168 | 3991 ± 85 |
| G, 80 °C | 3855 ± 59 | 497 ± 37 |
| S, 80 °C | 1807 ± 62 | 5786 ± 152 |
| G + S, 80 °C | 6024 ± 87 | 4874 ± 74 |
| Sample | H2S (ppm) | CH4 (%) | CO2 (%) |
|---|---|---|---|
| G, 38.6 °C | 397 ± 49 | 0.13 ± 0.03 | 15.43 ± 3.60 |
| G + R, 38.6 °C | 1744 ± 293 | 6.26 ± 0.65 | 26.16 ± 4.24 |
| S, 38.6 °C | 237 ± 24 | 7.30 ± 0.46 | 14.23 ± 1.15 |
| S + R, 38.6 °C | 181 ± 48 | 13.88 ± 2.09 | 12.78 ± 1.88 |
| G + S, 38.6 °C | 881 ± 182 | 4.25 ± 0.40 | 12.25 ± 2.87 |
| G + S + R, 38.6 °C | 1003 ± 140 | 5.15 ± 0.08 | 13.42 ± 3.06 |
| G, 80 °C | 22 ± 1 | 0.07 ± 0.02 | 2.06 ± 0.16 |
| S, 80 °C | 24 ± 7 | 0.08 ± 0.03 | 2.45 ± 0.58 |
| G+S, 80 °C | 18 ± 4 | 0.06 ± 0.02 | 1.43 ± 0.40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hren, R.; Petrovič, A.; Čuček, L.; Simonič, M. Determination of Various Parameters during Thermal and Biological Pretreatment of Waste Materials. Energies 2020, 13, 2262. https://doi.org/10.3390/en13092262
Hren R, Petrovič A, Čuček L, Simonič M. Determination of Various Parameters during Thermal and Biological Pretreatment of Waste Materials. Energies. 2020; 13(9):2262. https://doi.org/10.3390/en13092262
Chicago/Turabian StyleHren, Robert, Aleksandra Petrovič, Lidija Čuček, and Marjana Simonič. 2020. "Determination of Various Parameters during Thermal and Biological Pretreatment of Waste Materials" Energies 13, no. 9: 2262. https://doi.org/10.3390/en13092262
APA StyleHren, R., Petrovič, A., Čuček, L., & Simonič, M. (2020). Determination of Various Parameters during Thermal and Biological Pretreatment of Waste Materials. Energies, 13(9), 2262. https://doi.org/10.3390/en13092262