High-Capacity Dual-Electrolyte Aluminum–Air Battery with Circulating Methanol Anolyte
Abstract
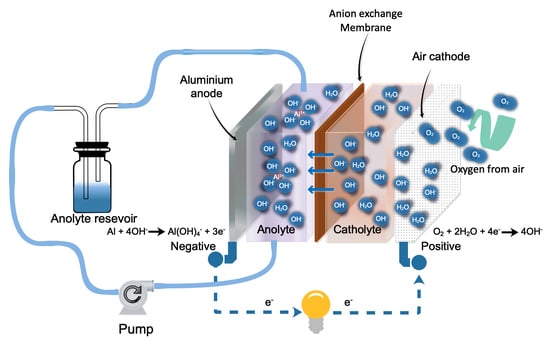
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrogen Evolution and Half-Cell Test
2.3. Full-Cell Test
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAB | aluminum–air battery |
| ZAB | zinc–air battery |
| HER | hydrogen evolution reaction |
| ORR | oxygen reduction reaction |
| EIS | electrochemical impedance spectroscopy |
| SEM | scanning electron microscope |
| AAFB | aluminum–air flow battery |
| OCV | open-circuit voltage |
| PTFE | polytetrafluoroethylene |
References
- Liu, Y.; Sun, Q.; Li, W.; Adair, K.R.; Li, J.; Sun, X. A comprehensive review on recent progress in aluminum–air batteries. Green Energy Environ. 2017, 2, 246–277. [Google Scholar] [CrossRef]
- Mokhtar, M.; Talib, M.Z.M.; Majlan, E.H.; Tasirin, S.M.; Ramli, W.M.F.W.; Daud, W.R.W.; Sahari, J. Recent developments in materials for aluminum–air batteries: A review. J. Ind. Eng. Chem. 2015, 32, 1–20. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J. Metal–Air Batteries: Will They Be the Future Electrochemical Energy Storage Device of Choice? ACS Energy Lett. 2017, 2, 1370–1377. [Google Scholar] [CrossRef]
- Rahman, M.A.; Wang, X.; Wen, C. High Energy Density Metal-Air Batteries: A Review. J. Electrochem. Soc. 2013, 160, A1759–A1771. [Google Scholar] [CrossRef]
- Lao-atiman, W.; Bumroongsil, K.; Arpornwichanop, A.; Bumroongsakulsawat, P.; Olaru, S.; Kheawhom, S. Model-Based Analysis of an Integrated Zinc-Air Flow Battery/Zinc Electrolyzer System. Front. Energy Res. 2019, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, B.J.; Shao-Horn, Y.; Hart, D.P. Suppressing corrosion in primary aluminum–air batteries via oil displacement. Science 2018, 362, 658. [Google Scholar] [CrossRef] [Green Version]
- Rashvand avei, M.; Jafarian, M.; Moghanni Bavil Olyaei, H.; Gobal, F.; Hosseini, S.M.; Mahjani, M.G. Study of the alloying additives and alkaline zincate solution effects on the commercial aluminum as galvanic anode for use in alkaline batteries. Mater. Chem. Phys. 2013, 143, 133–142. [Google Scholar] [CrossRef]
- Abedin, S.Z.E.; Endres, F. Electrochemical Behaviour of Al, Al-In and Al-Ga-In Alloys in Chloride Solutions Containing Zinc Ions. J. Appl. Electrochem. 2004, 34, 1071–1080. [Google Scholar] [CrossRef]
- Wang, D.; Li, H.; Liu, J.; Zhang, D.; Gao, L.; Tong, L. Evaluation of AA5052 alloy anode in alkaline electrolyte with organic rare-earth complex additives for aluminium-air batteries. J. Power Sources 2015, 293, 484–491. [Google Scholar] [CrossRef]
- Mori, R. Addition of Ceramic Barriers to Aluminum–Air Batteries to Suppress By-product Formation on Electrodes. J. Electrochem. Soc. 2015, 162, A288–A294. [Google Scholar] [CrossRef]
- Mori, R. A novel aluminium–Air rechargeable battery with Al2O3 as the buffer to suppress byproduct accumulation directly onto an aluminium anode and air cathode. RSC Adv. 2014, 4, 30346–30351. [Google Scholar] [CrossRef]
- Abdel-Gaber, A.M.; Khamis, E.; Abo-Eldahab, H.; Adeel, S. Novel package for inhibition of aluminium corrosion in alkaline solutions. Mater. Chem. Phys. 2010, 124, 773–779. [Google Scholar] [CrossRef]
- Liu, J.; Wang, D.; Zhang, D.; Gao, L.; Lin, T. Synergistic effects of carboxymethyl cellulose and ZnO as alkaline electrolyte additives for aluminium anodes with a view towards Al-air batteries. J. Power Sources 2016, 335, 1–11. [Google Scholar] [CrossRef]
- Mohamad, A.A. Electrochemical properties of aluminum anodes in gel electrolyte-based aluminum-air batteries. Corros. Sci. 2008, 50, 3475–3479. [Google Scholar] [CrossRef]
- Gelman, D.; Shvartsev, B.; Ein-Eli, Y. Aluminum–air battery based on an ionic liquid electrolyte. J. Mater. Chem. A 2014, 2, 20237–20242. [Google Scholar] [CrossRef]
- Zuo, Y.; Yu, Y.; Zuo, C.; Ning, C.; Liu, H.; Gu, Z.; Cao, Q.; Shen, C. Low-Temperature Performance of Al-air Batteries. Energies 2019, 12, 612. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Liu, F.; Wang, W.; Yang, G.; Zheng, D.; Wu, Z.; Leung, M.K.H. A high-capacity dual-electrolyte aluminum/air electrochemical cell. RSC Adv. 2014, 4, 30857–30863. [Google Scholar] [CrossRef]
- Koroleva, E.V.; Thompson, G.E.; Hollrigl, G.; Bloeck, M. Surface morphological changes of aluminium alloys in alkaline solution: Effect of second phase material. Corros. Sci. 1999, 41, 1475–1495. [Google Scholar] [CrossRef]
- Weon, J.-I.; Woo, H.-S. Corrosion mechanism of aluminum alloy by ethylene glycol-based solution. Mater. Corros. 2013, 64, 50–59. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, D.; Lee, K.; Gao, L. Performance of AA5052 alloy anode in alkaline ethylene glycol electrolyte with dicarboxylic acids additives for aluminium-air batteries. J. Power Sources 2015, 297, 464–471. [Google Scholar] [CrossRef]
- Wang, J.-B.; Wang, J.-M.; Shao, H.-B.; Zhang, J.-Q.; Cao, C.-N. The corrosion and electrochemical behaviour of pure aluminium in alkaline methanol solutions. J. Appl. Electrochem. 2007, 37, 753–758. [Google Scholar] [CrossRef]
- Wang, J.B.; Wang, J.M.; Shao, H.B.; Chang, X.T.; Wang, L.; Zhang, J.Q.; Cao, C.N. The corrosion and electrochemical behavior of pure aluminum in additive-containing alkaline methanol–water mixed solutions. Mater. Corros. 2009, 60, 269–273. [Google Scholar] [CrossRef]
- Ryu, J.; Jang, H.; Park, J.; Yoo, Y.; Park, M.; Cho, J. Seed-mediated atomic-scale reconstruction of silver manganate nanoplates for oxygen reduction towards high-energy aluminum-air flow batteries. Nat. Commun. 2018, 9, 3715. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Han, S.J.; Arponwichanop, A.; Yonezawa, T.; Kheawhom, S. Ethanol as an electrolyte additive for alkaline zinc-air flow batteries. Sci. Rep. 2018, 8, 11273. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, A.; Hosseini, S.; Somwangthanaroj, A.; Mohamad, A.A.; Kheawhom, S. Poly(2,6-Dimethyl-1,4-Phenylene Oxide)-Based Hydroxide Exchange Separator Membranes for Zinc–Air Battery. Int. J. Mol. Sci. 2019, 20, 3678. [Google Scholar] [CrossRef] [Green Version]
- Shinagawa, T.; Garcia-Esparza, A.T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Gawad, S.A.; Osman, W.M.; Fekry, A.M. Characterization and corrosion behavior of anodized Aluminum alloys for military industries applications in artificial seawater. Surf. Interfaces 2019, 14, 314–323. [Google Scholar] [CrossRef]
- Hosseini, S.; Lao-atiman, W.; Han, S.J.; Arpornwichanop, A.; Yonezawa, T.; Kheawhom, S. Discharge Performance of Zinc-Air Flow Batteries Under the Effects of Sodium Dodecyl Sulfate and Pluronic F-127. Sci. Rep. 2018, 8, 14909. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Gaber, A.M.; Khamis, E.; Abo-ElDahab, H.; Adeel, S. Inhibition of aluminium corrosion in alkaline solutions using natural compound. Mater. Chem. Phys. 2008, 109, 297–305. [Google Scholar] [CrossRef]
- Moghadam, Z.; Shabani-Nooshabadi, M.; Behpour, M. Electrochemical performance of aluminium alloy in strong alkaline media by urea and thiourea as inhibitor for aluminium-air batteries. J. Mol. Liq. 2017, 242, 971–978. [Google Scholar] [CrossRef]
- Verma, C.; Singh, P.; Bahadur, I.; Ebenso, E.E.; Quraishi, M.A. Electrochemical, thermodynamic, surface and theoretical investigation of 2-aminobenzene-1,3-dicarbonitriles as green corrosion inhibitor for aluminum in 0.5M NaOH. J. Mol. Liq. 2015, 209, 767–778. [Google Scholar] [CrossRef]
- Mori, R. All solid state rechargeable aluminum–air battery with deep eutectic solvent based electrolyte and suppression of byproducts formation. RSC Adv. 2019, 9, 22220–22226. [Google Scholar] [CrossRef] [Green Version]
- Avoundjian, A.; Galvan, V.; Gomez, F.A. An Inexpensive Paper-Based Aluminum-Air Battery. Micromachines 2017, 8, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Pan, W.; Kwok, H.; Lu, X.; Leung, D.Y.C. Low-cost Al-air batteries with paper-based solid electrolyte. Energy Procedia 2019, 158, 522–527. [Google Scholar] [CrossRef]






| % Water | Ecorr (V vs. Ag/AgCl) | Icorr (A·cm−2) | βa (V·dec−1) | βc (V·dec−1) | Rp (Ω) |
|---|---|---|---|---|---|
| 0% water | −1.4 | 0.002 | 1.05 | −6.18 | 252.31 |
| 5% water | −1.5 | 0.003 | 0.86 | −5.50 | 119.04 |
| 10% water | −1.5 | 0.004 | 0.71 | −4.88 | 61.11 |
| 20% water | −1.5 | 0.006 | 0.63 | −3.60 | 36.71 |
| % Water | Rs (Ω·cm2) | CPE1 (S·cm2 s−n) | R1 (Ω·cm2) | R2 (Ω·cm2) | CPE2 (S·cm2 s−n) | Ionic Conductivity (mS.cm−1) |
|---|---|---|---|---|---|---|
| 0% water | 2.23 | 19.38 | 39.76 | 13.05 | 6.82 | 29.24 |
| 5% water | 1.46 | 12.19 | 34.23 | 16.72 | 18.94 | 38.08 |
| 10% water | 1.23 | 10.16 | 23.26 | 6.68 | 7.18 | 46.19 |
| 20% water | 0.38 | 3.40 | 8.82 | 9.94 | 4.15 | 64.87 |
| % Water | Maximum Current Density (mA·cm−2) | Maximum Power Density (mW·cm−2) | Internal Resistance (Ω) |
|---|---|---|---|
| 0% | 15 | 7.5 | 76.70 |
| 5% | 18 | 8.5 | 65.70 |
| 10% | 30 | 15.4 | 39.24 |
| 20% | 36 | 19.6 | 33.09 |
| % Water | Discharge Voltage (V) | Specific Capacity (mAh/gAl) | Utilization Percentage | Discharge Time (hours) |
|---|---|---|---|---|
| 0% | 0.77 | 2328 | 78% | ~40 |
| 5% | 0.98 | 1700 | 57% | ~28 |
| 10% | 1.02 | 1130 | 38% | ~18 |
| 20% | 1.10 | 465 | 16% | ~7 |
| System | Air Cathode | Maximum Power Density (mW·cm−2) | Maximum Specific Capacity (mAh/gAl) | References |
|---|---|---|---|---|
| Dual-electrolyte AAFB (3 M KOH anhydrous methanol anolyte) | MnO2/C | 7.5 | 2328 | This work |
| Dual-electrolyte AAB (3 M KOH anhydrous methanol anolyte) | Pt/C | 28 | 1810 | [17] |
| Solid state rechargeable AAB | TiN | N/A | 35.8 | [32] |
| Paper-based AAB | C | 0.8 | N/A | [33] |
| Paper-based solid electrolyte AAB | Ag/C | 3.8 | 900.8 | [34] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teabnamang, P.; Kao-ian, W.; Nguyen, M.T.; Yonezawa, T.; Cheacharoen, R.; Kheawhom, S. High-Capacity Dual-Electrolyte Aluminum–Air Battery with Circulating Methanol Anolyte. Energies 2020, 13, 2275. https://doi.org/10.3390/en13092275
Teabnamang P, Kao-ian W, Nguyen MT, Yonezawa T, Cheacharoen R, Kheawhom S. High-Capacity Dual-Electrolyte Aluminum–Air Battery with Circulating Methanol Anolyte. Energies. 2020; 13(9):2275. https://doi.org/10.3390/en13092275
Chicago/Turabian StyleTeabnamang, Pemika, Wathanyu Kao-ian, Mai Thanh Nguyen, Tetsu Yonezawa, Rongrong Cheacharoen, and Soorathep Kheawhom. 2020. "High-Capacity Dual-Electrolyte Aluminum–Air Battery with Circulating Methanol Anolyte" Energies 13, no. 9: 2275. https://doi.org/10.3390/en13092275
APA StyleTeabnamang, P., Kao-ian, W., Nguyen, M. T., Yonezawa, T., Cheacharoen, R., & Kheawhom, S. (2020). High-Capacity Dual-Electrolyte Aluminum–Air Battery with Circulating Methanol Anolyte. Energies, 13(9), 2275. https://doi.org/10.3390/en13092275