The Impact of Nanofluids on Droplet/Spray Cooling of a Heated Surface: A Critical Review
Abstract
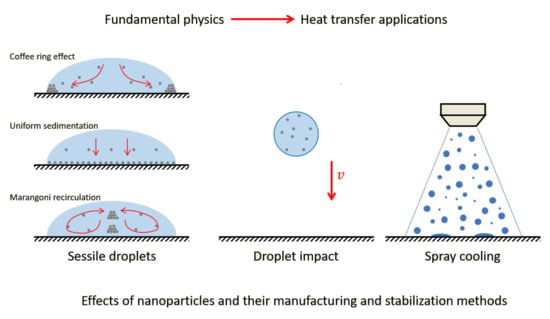
:1. Introduction
- 1
- During the initial stage, the droplet reaches its maximum diameter, which depends on both volume and advancing contact angle. Evaporation is neglected in this stage.
- 2
- In the first stage the droplet radius remains constant due to contact line pinning. Evaporation flattens the droplet profile.
- 3
- In the second stage, the contact line unpins and radius decreases while the contact angle stays constant.
- 4
- Lastly, during the final stage, both the contact angle and droplet radius decrease until the droplet completely disappears.
Motivation of the Review
2. Nanofluid Synthesis and Properties
2.1. Synthesis of Nanofluids
2.1.1. One-Step Method
2.1.2. Two-Step Method
2.1.3. Stabilization Mechanisms
2.2. Effects of Additional Stabilization Mechanisms on Droplet Dynamics Behavior
3. Nanofluid Droplet Behavior
3.1. Evaporation of Nanofluids Sessile Droplets
3.2. Splashing, Spreading and Heat Transfer Enhancement of Nanofluids
4. Nanofluids Spray Cooling
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviations: | ||
| AAO | Anodic Aluminum Oxide | |
| CHF | Critical heat flux | |
| DLVO | Derjaguin-Landau-Verwey-Overbeek | |
| DWCNT | Double-walled carbon nanotubes | |
| EG | Ethylene glycol | |
| LDH | Layered double hydroxide | |
| MWCNT | Multi-walled carbon nanotubes | |
| SWCNT | Single-walled carbon nanotubes | |
| Non-dimensional numbers: | ||
| Oh | Ohnesorge number | |
| Re | Reynolds number | |
| We | Weber number | |
| Parameters: | ||
| Spread factor | [-] | |
| Maximum spread factor | [-] | |
| Dynamic viscosity | [Pa·s] | |
| Viscosity of medium | [Pa·s] | |
| Viscosity of nanofluid | [Pa·s] | |
| Relative viscosity | [-] | |
| Surface tension between liquid phase and gas phase | [N/m] | |
| Surface tension between solid phase and gas phase | [N/m] | |
| Surface tension between solid phase and liquid phase | [N/m] | |
| Weight fraction | [-] | |
| Volume fraction | [-] | |
| Effective volume fractions of clusters | [-] | |
| Maximum particle loading | [-] | |
| Droplet density | [kg/m] | |
| Medium density | [kg/m] | |
| Nanofluid density | [kg/m] | |
| Nanoparticle density | [kg/m] | |
| Surface tension | [N/m] | |
| Medium heat capacity | [J/K] | |
| Nanofluid heat capacity | [J/K] | |
| Nanoparticle heat capacity | [J/K] | |
| Droplet diameter before impact | [m] | |
| Maximum spreading diameter of droplet | [m] | |
| h | Non-locality characteristic length | [m] |
| K | Splashing constant | [-] |
| Thermal conductivity of medium | [W/(mK)] | |
| Effective thermal conductivity of nanofluid | [W/(mK)] | |
| Thermal conductivity of nanoparticles | [W/(mK)] | |
| R | Particle size | [m] |
| Droplet impact speed | [m/s] | |
| S | Spreading parameter | [N/m] |
References
- Liang, G.; Mudawar, I. Review of drop impact on heated walls. Int. J. Heat Mass Transf. 2017, 106, 103–126. [Google Scholar] [CrossRef]
- Almohammadi, H.; Amirfazli, A. Droplet impact: Viscosity and wettability effects on splashing. J. Colloid Interface Sci. 2019, 553, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Guggilla, G.; Narayanaswamy, R.; Pattamatta, A. An experimental investigation into the spread and heat transfer dynamics of a train of two concentric impinging droplets over a heated surface. Exp. Therm. Fluid Sci. 2020, 110, 109916. [Google Scholar] [CrossRef]
- Marengo, M.; Antonini, C.; Roisman, I.V.; Tropea, C. Drop collisions with simple and complex surfaces. Curr. Opin. Colloid Interface Sci. 2011, 16, 292–302. [Google Scholar] [CrossRef]
- Chaze, W.; Caballina, O.; Castanet, G.; Pierson, J.F.; Lemoine, F.; Maillet, D. Heat flux reconstruction by inversion of experimental infrared temperature measurements—Application to the impact of a droplet in the film boiling regime. Int. J. Heat Mass Transf. 2019, 128, 469–478. [Google Scholar] [CrossRef]
- Murshed, S.S.; de Castro, C.N. A critical review of traditional and emerging techniques and fluids for electronics cooling. Renew. Sustain. Energy Rev. 2017, 78, 821–833. [Google Scholar] [CrossRef]
- Deng, W.; Gomez, A. Electrospray cooling for microelectronics. Int. J. Heat Mass Transf. 2011, 54, 2270–2275. [Google Scholar] [CrossRef]
- Yu, W.; France, D.M.; Routbort, J.L.; Choi, S.U.S. Review and Comparison of Nanofluid Thermal Conductivity and Heat Transfer Enhancements. Heat Transf. Eng. 2008, 29, 432–460. [Google Scholar] [CrossRef]
- Rioboo, R.; Tropea, C.; Marengo, M. Outcomes from a drop impact on solid surfaces. At. Sprays 2001, 11, 155–165. [Google Scholar] [CrossRef]
- Roisman, I.V.; Rioboo, R.; Tropea, C. Normal impact of a liquid drop on a dry surface: Model for spreading and receding. Proc. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2002, 458, 1411–1430. [Google Scholar] [CrossRef]
- Yarin, A. Drop impact dynamics: Splashing, Spreading, Receding, Bouncing. Annu. Rev. Fluid Mech. 2006, 38, 159–192. [Google Scholar] [CrossRef]
- Josserand, C.; Thoroddsen, S. Drop Impact on a Solid Surface. Annu. Rev. Fluid Mech. 2016, 48, 365–391. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.; Lourenço, S.D.N.; Yan, W.M. Lattice Boltzmann simulation of droplet dynamics on granular surfaces with variable wettability. Phys. Rev. E 2018, 98, 012902. [Google Scholar] [CrossRef] [PubMed]
- Herbaut, R.; Brunet, P.; Limat, L.; Royon, L. Liquid spreading on cold surfaces: Solidification-induced stick-slip dynamics. Phys. Rev. Fluids 2019, 4, 033603. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Li, J.; Guo, P.; Lv, Q. Experimental studies on the effect of Reynolds and Weber numbers on the impact forces of low-speed droplets colliding with a solid surface. Exp. Fluids 2017, 58, 125. [Google Scholar] [CrossRef]
- Chandra, S.; Avedisian, C.T. On the collision of a droplet with a solid surface. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1991, 432, 13–41. [Google Scholar] [CrossRef]
- Pasandideh-Fard, M.; Qiao, Y.M.; Chandra, S.; Mostaghimi, J. Capillary effects during droplet impact on a solid surface. Phys. Fluids 1996, 8, 650–659. [Google Scholar] [CrossRef]
- Mao, T.; Kuhn, D.C.S.; Tran, H. Spread and rebound of liquid droplets upon impact on flat surfaces. AIChE J. 1997, 43, 2169–2179. [Google Scholar] [CrossRef]
- Liu, J.; Franco, W.; Aguilar, G. Effect of Surface Roughness on Single Cryogen Droplet Spreading. J. Phys. Condens. Matter 2008, 130, 041402. [Google Scholar] [CrossRef]
- Wang, F.; Yang, L.; Wang, L.; Zhu, Y.; Fang, T. Maximum Spread of Droplet Impacting onto Solid Surfaces with Different Wettabilities: Adopting a Rim–Lamella Shape. Langmuir 2019, 35, 3204–3214. [Google Scholar] [CrossRef]
- Akao, F.; Araki, K.; Mori, S.; Moriyama, A. Deformation Behaviors of a Liquid Droplet Impinging onto Hot Metal Surface. Trans. Iron Steel Inst. Jpn. 1980, 20, 737–743. [Google Scholar] [CrossRef]
- Castanet, G.; Caballina, O.; Labergue, A.; Gradeck, M.; Lemoine, F. Effect of the Vapor Flow on the Drop Spreading in the Leidenfrost Regime. In International Heat Transfer Conference Digital Library; Begel House Inc.: Danbury, CT, USA, 2014. [Google Scholar] [CrossRef]
- Tran, T.; Staat, H.J.J.; Prosperetti, A.; Sun, C.; Lohse, D. Drop Impact on Superheated Surfaces. Phys. Rev. Lett. 2012, 108, 036101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikalo, S.; Marengo, M.; Tropea, C.; Ganic, E. Analysis of impact of droplets on horizontal surfaces. Exp. Therm. Fluid Sci. 2002, 25, 503–510. [Google Scholar] [CrossRef]
- Fedorchenko, A.I.; Wang, A.B.; Wang, Y.H. Effect of capillary and viscous forces on spreading of a liquid drop impinging on a solid surface. Phys. Fluids 2005, 17, 093104. [Google Scholar] [CrossRef] [Green Version]
- Clanet, C.; Beguin, C.; Richard, D.; Quere, D. Maximal deformation of an impacting drop. J. Fluid Mech. 2004, 517, 199–208. [Google Scholar] [CrossRef]
- Castanet, G.; Caballina, O.; Lemoine, F. Drop spreading at the impact in the Leidenfrost boiling. Phys. Fluids 2015, 27, 063302. [Google Scholar] [CrossRef] [Green Version]
- Scheller, B.L.; Bousfield, D.W. Newtonian drop impact with a solid surface. AIChE J. 1995, 41, 1357–1367. [Google Scholar] [CrossRef]
- Moon, J.H.; Cho, M.; Lee, S.H. Dynamic wetting and heat transfer characteristics of a liquid droplet impinging on heated textured surfaces. Int. J. Heat Mass Transf. 2016, 97, 308–317. [Google Scholar] [CrossRef]
- Mundo, C.; Sommerfeld, M.; Tropea, C. Droplet-wall collisions: Experimental studies of the deformation and breakup process. Int. J. Multiph. Flow 1995, 21, 151–173. [Google Scholar] [CrossRef]
- Wal, R.L.V.; Berger, G.M.; Mozes, S.D. The splash/non-splash boundary upon a dry surface and thin fluid film. Exp. Fluids 2006, 40, 53–59. [Google Scholar] [CrossRef]
- Lagubeau, G.; Fontelos, M.A.; Josserand, C.; Maurel, A.; Pagneux, V.; Petitjeans, P. Spreading dynamics of drop impacts. J. Fluid Mech. 2012, 713, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Bird, J.C.; Tsai, S.S.H.; Stone, H.A. Inclined to splash: Triggering and inhibiting a splash with tangential velocity. New J. Phys. 2009, 11, 063017. [Google Scholar] [CrossRef]
- Moreira, A.; Moita, A.; Panao, M. Advances and challenges in explaining fuel spray impingement: How much of single droplet impact research is useful? Prog. Energy Combust. Sci. 2010, 36, 554–580. [Google Scholar] [CrossRef]
- Pan, K.L.; Tseng, K.C.; Wang, C.H. Breakup of a droplet at high velocity impacting a solid surface. Exp. Fluids 2010, 48, 143–156. [Google Scholar] [CrossRef]
- Palacios, J.; Hernandez, J.; Gomez, P.; Zanzi, C.; Lopez, J. Experimental study of splashing patterns and the splashing/deposition threshold in drop impacts onto dry smooth solid surfaces. Exp. Therm. Fluid Sci. 2013, 44, 571–582. [Google Scholar] [CrossRef]
- Riboux, G.; Gordillo, J.M. Experiments of Drops Impacting a Smooth Solid Surface: A Model of the Critical Impact Speed for Drop Splashing. Phys. Rev. Lett. 2014, 113, 024507. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, W.W.; Nagel, S.R. Drop Splashing on a Dry Smooth Surface. Phys. Rev. Lett. 2005, 94, 184505. [Google Scholar] [CrossRef] [Green Version]
- Tang, C.; Qin, M.; Weng, X.; Zhang, X.; Zhang, P.; Li, J.; Huang, Z. Dynamics of droplet impact on solid surface with different roughness. Int. J. Multiph. Flow 2017, 96, 56–69. [Google Scholar] [CrossRef]
- Hu, H.; Larson, R.G. Evaporation of a Sessile Droplet on a Substrate. J. Phys. Chem. B 2002, 106, 1334–1344. [Google Scholar] [CrossRef]
- Rymkiewicz, J.; Zapalowicz, Z. Analysis of the evaporation process for water droplet on flat heated surface. Int. Commun. Heat Mass Transf. 1993, 20, 687–697. [Google Scholar] [CrossRef]
- Semenov, S.; Trybala, A.; Rubio, R.G.; Kovalchuk, N.; Starov, V.; Velarde, M.G. Simultaneous spreading and evaporation: Recent developments. Adv. Colloid Interface Sci. 2014, 206, 382–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Choi, S.; Li, S.; Eastman, J. Measuring Thermal Conductivity of Fluids Containing Oxide Nanoparticles. J. Heat Transf. 1999, 121, 280–289. [Google Scholar] [CrossRef]
- Léal, L.; Miscevic, M.; Lavieille, P.; Amokrane, M.; Pigache, F.; Topin, F.; Nogarède, B.; Tadrist, L. An overview of heat transfer enhancement methods and new perspectives: Focus on active methods using electroactive materials. Int. J. Heat Mass Transf. 2013, 61, 505–524. [Google Scholar] [CrossRef]
- Yang, L.; Xu, J.; Du, K.; Zhang, X. Recent developments on viscosity and thermal conductivity of nanofluids. Powder Technol. 2017, 317, 348–369. [Google Scholar] [CrossRef]
- Murshed, S.S.; de Castro, C.N. Boiling Heat Transfer and Droplet Spreading of Nanofluids. Recent Patents Nanotechnol. 2013, 7, 216–223. [Google Scholar] [CrossRef]
- Heris, S.Z.; Etemad, S.; Esfahany, M.N. Experimental investigation of oxide nanofluids laminar flow convective heat transfer. Int. Commun. Heat Mass Transf. 2006, 33, 529–535. [Google Scholar] [CrossRef]
- Gorji, T.B.; Ranjbar, A.A. A review on optical properties and application of nanofluids in direct absorption solar collectors (DASCs). Renew. Sustain. Energy Rev. 2017, 72, 10–32. [Google Scholar] [CrossRef]
- Taylor, R.; Coulombe, S.; Otanicar, T.; Phelan, P.; Gunawan, A.; Lv, W.; Rosengarten, G.; Prasher, R.; Tyagi, H. Small particles, big impacts: A review of the diverse applications of nanofluids. J. Appl. Phys. 2013, 113, 011301. [Google Scholar] [CrossRef]
- Azmi, W.; Sharma, K.; Sarma, P.; Mamat, R.; Anuar, S.; Rao, V.D. Experimental determination of turbulent forced convection heat transfer and friction factor with SiO2 nanofluid. Exp. Therm. Fluid Sci. 2013, 51, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Bandarra Filho, E.P.; Thome, J.R. Nanofluid Two-Phase Flow and Thermal Physics: A New Research Frontier of Nanotechnology and Its Challenges. J. Nanosci. Nanotechnol. 2008, 8, 3315–3332. [Google Scholar] [CrossRef]
- Bang, I.C.; Heung Chang, S. Boiling heat transfer performance and phenomena of Al2O3-water nano-fluids from a plain surface in a pool. Int. J. Heat Mass Transf. 2005, 48, 2407–2419. [Google Scholar] [CrossRef]
- Kim, S.; Bang, I.; Buongiorno, J.; Hu, L. Surface wettability change during pool boiling of nanofluids and its effect on critical heat flux. Int. J. Heat Mass Transf. 2007, 50, 4105–4116. [Google Scholar] [CrossRef]
- Kim, H.D.; Kim, M.H. Effect of nanoparticle deposition on capillary wicking that influences the critical heat flux in nanofluids. Appl. Phys. Lett. 2007, 91, 014104. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, J.C. A Treatise on Electricity and Magnetism; Clarendon Press: London, UK, 1873; Volume 2. [Google Scholar]
- Xue, Q.Z. Model for effective thermal conductivity of nanofluids. Phys. Lett. A 2003, 307, 313–317. [Google Scholar] [CrossRef]
- Kumar, D.H.; Patel, H.E.; Kumar, V.R.R.; Sundararajan, T.; Pradeep, T.; Das, S.K. Model for Heat Conduction in Nanofluids. Phys. Rev. Lett. 2004, 93, 144301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, S.; Chauhan, K.P.; Kanagaraj, S. Modeling of thermal conductivity of nanofluids by modifying Maxwell’s equation using cell model approach. J. Nanoparticle Res. 2011, 13, 2791–2798. [Google Scholar] [CrossRef]
- Shaker, M.; Birgersson, E.; Mujumdar, A. Extended Maxwell model for the thermal conductivity of nanofluids that accounts for nonlocal heat transfer. Int. J. Therm. Sci. 2014, 84, 260–266. [Google Scholar] [CrossRef]
- Hamilton, R.L.; Crosser, O.K. Thermal conductivity of heterogeneous two-component systems. Ind. Eng. Chem. Fundam. 1962, 1, 187–191. [Google Scholar] [CrossRef]
- Kakac, S.; Pramuanjaroenkij, A. Review of convective heat transfer enhancement with nanofluids. Int. J. Heat Mass Transf. 2009, 52, 3187–3196. [Google Scholar] [CrossRef]
- Eastman, J.; Phillpot, S.; Choi, S.; Keblinski, P. Thermal transport in nanofluids. Annu. Rev. Mater. Res. 2004, 34, 219–246. [Google Scholar] [CrossRef]
- Rea, U.; McKrell, T.; wen Hu, L.; Buongiorno, J. Laminar convective heat transfer and viscous pressure loss of alumina-water and zirconia-water nanofluids. Int. J. Heat Mass Transf. 2009, 52, 2042–2048. [Google Scholar] [CrossRef]
- Maiga, S.E.B.; Nguyen, C.T.; Galanis, N.; Roy, G. Heat transfer behaviours of nanofluids in a uniformly heated tube. Superlattices Microstruct. 2004, 35, 543–557. [Google Scholar] [CrossRef]
- Chow, T.S. Viscosities of concentrated dispersions. Phys. Rev. E 1993, 48, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, G.K.; Green, J.T. The hydrodynamic interaction of two small freely-moving spheres in a linear flow field. J. Fluid Mech. 1972, 56, 375–400. [Google Scholar] [CrossRef]
- Batchelor, G.K. The effect of Brownian motion on the bulk stress in a suspension of spherical particles. J. Fluid Mech. 1977, 83, 97–117. [Google Scholar] [CrossRef]
- Prasher, R.; Song, D.; Wang, J.; Phelan, P. Measurements of nanofluid viscosity and its implications for thermal applications. Appl. Phys. Lett. 2006, 89, 133108. [Google Scholar] [CrossRef]
- Krieger, I.M.; Dougherty, T.J. A Mechanism for Non-Newtonian Flow in Suspensions of Rigid Spheres. Trans. Soc. Rheol. 1959, 3, 137–152. [Google Scholar] [CrossRef]
- de Rooij, R.; Potanin, A.A.; van den Ende, D.; Mellema, J. Steady shear viscosity of weakly aggregating polystyrene latex dispersions. J. Chem. Phys. 1993, 99, 9213–9223. [Google Scholar] [CrossRef]
- Selvakumar, R.D.; Dhinakaran, S. Effective viscosity of nanofluids—A modified Krieger-Dougherty model based on particle size distribution (PSD) analysis. J. Mol. Liq. 2017, 225, 20–27. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Tan, S.H.; Nguyen, N.T. Temperature dependence of interfacial properties and viscosity of nanofluids for droplet-based microfluidics. J. Phys. D Appl. Phys. 2008, 41, 085502. [Google Scholar] [CrossRef]
- Tanvir, S.; Qiao, L. Surface tension of Nanofluid-type fuels containing suspended nanomaterials. Nanoscale Res. Lett. 2012, 7, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, H.; Striolo, A. Nanoparticle effects on the water-oil interfacial tension. Phys. Rev. E 2012, 86, 051610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, D.; Arasu, A. A review on preparation, characterization, properties and applications of nanofluids. Renew. Sustain. Energy Rev. 2016, 60, 21–40. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Eastman, J.A. Enhanced Heat Transfer Using Nanofluids. U.S. Patent 6,221,275 B1, 24 April 2001. [Google Scholar]
- Chang, H.; Tsung, T.T.; Yang, Y.C.; Chen, L.C.; Lin, H.M.; Lin, C.K.; Jwo, C.S. Nanoparticle suspension preparation using the arc spray nanoparticle synthesis system combined with ultrasonic vibration and rotating electrode. Int. J. Adv. Manuf. Technol. 2005, 26, 552–558. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sengupta, I.; Sarkar, I.; Pal, S.K.; Chakraborty, S. Effect of surfactant on thermo-physical properties and spray cooling heat transfer performance of Cu-Zn-Al LDH nanofluid. Appl. Clay Sci. 2019, 168, 43–55. [Google Scholar] [CrossRef]
- Joseph, A.; Xavier, M.M.; Fal, J.; Żyła, G.; Sasi, S.; Radhakrishnan Nair, P.; Padmanabhan, A.S.; Mathew, S. Synthesis and electrochemical characterization of electroactive IoNanofluids with high dielectric constants from hydrated ferrous sulphate. Chem. Commun. 2019, 55, 83–86. [Google Scholar] [CrossRef]
- Rueda-García, D.; Rodríguez-Laguna, M.D.R.; Chávez-Angel, E.; Dubal, D.P.; Cabán-Huertas, Z.; Benages-Vilau, R.; Gómez-Romero, P. From Thermal to Electroactive Graphene Nanofluids. Energies 2019, 12, 4545. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhang, X.; Su, M. Synthesis and thermal stability of Field’s alloy nanoparticles and nanofluid. Mater. Lett. 2017, 205, 6–9. [Google Scholar] [CrossRef]
- Wei, X.; Wang, L. Synthesis and thermal conductivity of microfluidic copper nanofluids. Particuology 2010, 8, 262–271. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sarkar, I.; Haldar, K.; Pal, S.K.; Chakraborty, S. Synthesis of Cu–Al layered double hydroxide nanofluid and characterization of its thermal properties. Appl. Clay Sci. 2015, 107, 98–108. [Google Scholar] [CrossRef]
- Xie, H.; Wang, J.; Xi, T.; Liu, Y.; Ai, F.; Wu, Q. Thermal conductivity enhancement of suspensions containing nanosized alumina particles. J. Appl. Phys. 2002, 91, 4568–4572. [Google Scholar] [CrossRef]
- Li, X.; Zou, C.; Lei, X.; Li, W. Stability and enhanced thermal conductivity of ethylene glycol-based SiC nanofluids. Int. J. Heat Mass Transf. 2015, 89, 613–619. [Google Scholar] [CrossRef]
- Li, C.H.; Peterson, G.P. Experimental investigation of temperature and volume fraction variations on the effective thermal conductivity of nanoparticle suspensions (nanofluids). J. Appl. Phys. 2006, 99, 084314. [Google Scholar] [CrossRef]
- Liu, Z.H.; Qiu, Y.H. Boiling heat transfer characteristics of nanofluids jet impingement on a plate surface. Heat Mass Transf. 2007, 43, 699–706. [Google Scholar] [CrossRef]
- Powell, C.; Fenwick, N.; Bresme, F.; Quirke, N. Wetting of nanoparticles and nanoparticle arrays. Colloids Surf. A Physicochem. Eng. Asp. 2002, 206, 241–251. [Google Scholar] [CrossRef]
- Mi, J.; He, Y.; Zhong, C. Theoretical study of wetting behavior of nanoparticles at fluid interfaces. AIChE J. 2009, 55, 747–755. [Google Scholar] [CrossRef]
- Zhang, F. Wettability on nAnoparticle Modified Surface: For Thermal Engineering. Ph.D. Thesis, University of Illinois at Urbana-Champaign, Urbana, IL, USA, 2017. [Google Scholar]
- Wen, D.; Lin, G.; Vafaei, S.; Zhang, K. Review of nanofluids for heat transfer applications. Particuology 2009, 7, 141–150. [Google Scholar] [CrossRef]
- Schroyen, B.; Swan, J.W.; Van Puyvelde, P.; Vermant, J. Quantifying the dispersion quality of partially aggregated colloidal dispersions by high frequency rheology. Soft Matter 2017, 13, 7897–7906. [Google Scholar] [CrossRef] [Green Version]
- Tatake, P.A.; Pandit, A.B. Modelling and experimental investigation into cavity dynamics and cavitational yield: Influence of dual frequency ultrasound sources. Chem. Eng. Sci. 2002, 57, 4987–4995. [Google Scholar] [CrossRef]
- Mandzy, N.; Grulke, E.; Druffel, T. Breakage of TiO2 agglomerates in electrostatically stabilized aqueous dispersions. Powder Technol. 2005, 160, 121–126. [Google Scholar] [CrossRef]
- Cronholm, P.; Midander, K.; Karlsson, H.L.; Elihn, K.; Wallinder, I.O.; Möller, L. Effect of sonication and serum proteins on copper release from copper nanoparticles and the toxicity towards lung epithelial cells. Nanotoxicology 2011, 5, 269–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taurozzi, J.; Hackley, V.; Wiesner, M. Preparation of nanoparticle dispersions from powdered material using ultrasonic disruption. NIST Special Publication 1200-2. NIST Spec. Publ. 2012, 1200-2. [Google Scholar] [CrossRef]
- Kaur, I.; Ellis, L.J.; Romer, I.; Tantra, R.; Carriere, M.; Allard, S.; Mayne-L’Hermite, M.; Minelli, C.; Unger, W.; Potthoff, A.; et al. Dispersion of Nanomaterials in Aqueous Media: Towards Protocol Optimization. JoVE 2017, e56074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Z.G.; Chen, J.F.; Zou, H.K.; Yun, J. Rheology of colloidal nanosized BaTiO3 suspension with ammonium salt of polyacrylic acid as a dispersant. Colloids Surf. A Physicochem. Eng. Asp. 2004, 244, 61–66. [Google Scholar] [CrossRef]
- ZielińAski, P.; Schulz, R.; Kaliaguine, S.; Van Neste, A. Structural transformations of alumina by high energy ball milling. J. Mater. Res. 1993, 8, 2985–2992. [Google Scholar] [CrossRef]
- Chikhalia, V.; Forbes, R.; Storey, R.; Ticehurst, M. The effect of crystal morphology and mill type on milling induced crystal disorder. Eur. J. Pharm. Sci. 2006, 27, 19–26. [Google Scholar] [CrossRef]
- Timofeeva, E.V.; Smith, D.S.; Yu, W.; France, D.M.; Singh, D.; Routbort, J.L. Particle size and interfacial effects on thermo-physical and heat transfer characteristics of water-based α-SiC nanofluids. Nanotechnology 2010, 21, 215703. [Google Scholar] [CrossRef]
- Menon, M.; Decourcelle, S.; Ramousse, S.; Larsen, P.H. Stabilization of Ethanol-Based Alumina Suspensions. J. Am. Ceram. Soc. 2006, 89, 457–464. [Google Scholar] [CrossRef]
- Das, K.K.; Somasundaran, P. Flocculation-dispersion characteristics of alumina using a wide molecular weight range of polyacrylic acids. Colloids Surf. A Physicochem. Eng. Asp. 2003, 223, 17–25. [Google Scholar] [CrossRef]
- Bondarenko, B.I.; Moraru, V.N.; Sidorenko, S.V.; Komysh, D.V.; Khovavko, A.I. Nanofluids for energetics: Effect of stabilization on the critical heat flux at boiling. Tech. Phys. Lett. 2012, 38, 856–860. [Google Scholar] [CrossRef]
- Zhu, D.; Li, X.; Wang, N.; Wang, X.; Gao, J.; Li, H. Dispersion behavior and thermal conductivity characteristics of Al2O3 –H2O nanofluids. Curr. Appl. Phys. 2009, 9, 131–139. [Google Scholar] [CrossRef]
- Furusawa, K.; Ueda, M.; Nashima, T. Bridging and depletion flocculation of synthetic latices induced by polyelectrolytes. Colloids Surf. A Physicochem. Eng. Asp. 1999, 153, 575–581. [Google Scholar] [CrossRef]
- Mewis, J.; Wagner, N.J. Colloidal Suspension Rheology; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Sen, S.; Govindarajan, V.; Pelliccione, C.J.; Wang, J.; Miller, D.J.; Timofeeva, E.V. Surface Modification Approach to TiO2 Nanofluids with High Particle Concentration, Low Viscosity, and Electrochemical Activity. ACS Appl. Mater. Interfaces 2015, 7, 20538–20547. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Z.h. A Kind of Nanofluid Consisting of Surface-Functionalized Nanoparticles. Nanoscale Res. Lett. 2010, 5, 1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NPCS Board of Consultants & Engineers. The Complete Book On Water Soluble Polymers; Asia Pacific Business Press Inc.: New Delhi, India, 2009. [Google Scholar]
- Croucher, M.D.; Hair, M.L. Upper and Lower Critical Flocculation Temperatures in Sterically Stabilized Nonaqueous Dispersions. Macromolecules 1978, 11, 874–879. [Google Scholar] [CrossRef]
- Stokes, R.; Evans, D. Fundamentals of Interfacial Engineering; Advances in Interfacial Engineering Series; Wiley-VCH: Hoboken, NJ, USA, 1997. [Google Scholar]
- Sarkar, I.; Behera, D.K.; Jha, J.M.; Pal, S.K.; Chakraborty, S. Effect of polymer additive on the cooling rate of a hot steel plate by using water jet. Exp. Therm. Fluid Sci. 2016, 70, 105–114. [Google Scholar] [CrossRef]
- Maranzano, B.J.; Wagner, N.J. Thermodynamic properties and rheology of sterically stabilized colloidal dispersions. Rheol. Acta 2000, 39, 483–494. [Google Scholar] [CrossRef]
- Li, X.; Zou, C.; Wang, T.; Lei, X. Rheological behavior of ethylene glycol-based SiC nanofluids. Int. J. Heat Mass Transf. 2015, 84, 925–930. [Google Scholar] [CrossRef]
- Ohtsuka, H.; Mizutani, H.; IIO, S.; Asai, K.; Kiguchi, T.; Satone, H.; Mori, T.; Tsubaki, J. Effects of sintering additives on dispersion properties of Al2O3 slurry containing polyacrylic acid dispersant. J. Eur. Ceram. Soc. 2011, 31, 517–522. [Google Scholar] [CrossRef]
- Nguyen, V.X.; Stebe, K.J. Patterning of Small Particles by a Surfactant-Enhanced Marangoni-Bénard Instability. Phys. Rev. Lett. 2002, 88, 164501. [Google Scholar] [CrossRef]
- Truskett, V.N.; Stebe, K.J. Influence of Surfactants on an Evaporating Drop: Fluorescence Images and Particle Deposition Patterns. Langmuir 2003, 19, 8271–8279. [Google Scholar] [CrossRef]
- Osman, A.; Shahidzadeh, N.; Stitt, H.; Shokri, N. Morphological transformations during drying of surfactant-nanofluid droplets. J. Ind. Eng. Chem. 2018, 67, 92–98. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Fang, X.; Somasundaran, P.; Attinger, D. Self-Assembly of Colloidal Particles from Evaporating Droplets: Role of DLVO Interactions and Proposition of a Phase Diagram. Langmuir 2010, 26, 7833–7842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevalier, Y.; Bolzinger, M.A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Zhong, X.; Crivoi, A.; Duan, F. Sessile nanofluid droplet drying. Adv. Colloid Interface Sci. 2015, 217, 13–30. [Google Scholar] [CrossRef]
- Sefiane, K.; Bennacer, R. Nanofluids droplets evaporation kinetics and wetting dynamics on rough heated substrates. Adv. Colloid Interface Sci. 2009, 147–148, 263–271. [Google Scholar] [CrossRef]
- Sefiane, K.; Skilling, J.; MacGillivray, J. Contact line motion and dynamic wetting of nanofluid solutions. Adv. Colloid Interface Sci. 2008, 138, 101–120. [Google Scholar] [CrossRef]
- Chen, R.H.; Phuoc, T.X.; Martello, D. Effects of nanoparticles on nanofluid droplet evaporation. Int. J. Heat Mass Transf. 2010, 53, 3677–3682. [Google Scholar] [CrossRef]
- Moghiman, M.; Aslani, B. Influence of nanoparticles on reducing and enhancing evaporation mass transfer and its efficiency. Int. J. Heat Mass Transf. 2013, 61, 114–118. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Shen, X.; Ho, C.M.; Wong, T.S. Minimal Size of Coffee Ring Structure. J. Phys. Chem. B 2010, 114, 5269–5274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mampallil, D.; Eral, H.B. A review on suppression and utilization of the coffee-ring effect. Adv. Colloid Interface Sci. 2018, 252, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Chon, C.H.; Paik, S.; Tipton, J.B.; Kihm, K.D. Effect of Nanoparticle Sizes and Number Densities on the Evaporation and Dryout Characteristics for Strongly Pinned Nanofluid Droplets. Langmuir 2007, 23, 2953–2960. [Google Scholar] [CrossRef] [PubMed]
- Moffat, J.R.; Sefiane, K.; Shanahan, M.E.R. Effect of TiO2 Nanoparticles on Contact Line Stick-Slip Behavior of Volatile Drops. J. Phys. Chem. B 2009, 113, 8860–8866. [Google Scholar] [CrossRef]
- Askounis, A.; Orejon, D.; Koutsos, V.; Sefiane, K.; Shanahan, M.E.R. Nanoparticle deposits near the contact line of pinned volatile droplets: Size and shape revealed by atomic force microscopy. Soft Matter 2011, 7, 4152–4155. [Google Scholar] [CrossRef]
- Orejon, D.; Sefiane, K.; Shanahan, M.E.R. Stick-Slip of Evaporating Droplets: Substrate Hydrophobicity and Nanoparticle Concentration. Langmuir 2011, 27, 12834–12843. [Google Scholar] [CrossRef]
- Askounis, A.; Sefiane, K.; Koutsos, V.; Shanahan, M.E.R. Structural transitions in a ring stain created at the contact line of evaporating nanosuspension sessile drops. Phys. Rev. E 2013, 87, 012301, Erratum in 2013, 88, 049903. [Google Scholar] [CrossRef] [Green Version]
- Askounis, A.; Sefiane, K.; Koutsos, V.; Shanahan, M.E. The effect of evaporation kinetics on nanoparticle structuring within contact line deposits of volatile drops. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 855–866. [Google Scholar] [CrossRef]
- Orejon, D.; Shanahan, M.E.R.; Takata, Y.; Sefiane, K. Kinetics of Evaporation of Pinned Nanofluid Volatile Droplets at Subatmospheric Pressures. Langmuir 2016, 32, 5812–5820. [Google Scholar] [CrossRef]
- Wasan, D.T.; Nikolov, A.D. Spreading of nanofluids on solids. Nature 2003, 423, 156–159. [Google Scholar] [CrossRef]
- Kondiparty, K.; Nikolov, A.D.; Wasan, D.; Liu, K.L. Dynamic Spreading of Nanofluids on Solids. Part I: Experimental. Langmuir 2012, 28, 14618–14623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shan, Y.; Li, L.; Lu, M.; Li, R. Modeling the self-assembly of nanoparticles into branched aggregates from a sessile nanofluid droplet. Appl. Therm. Eng. 2016, 94, 650–656. [Google Scholar] [CrossRef]
- Wasan, D.; Nikolov, A.; Kondiparty, K. The wetting and spreading of nanofluids on solids: Role of the structural disjoining pressure. Curr. Opin. Colloid Interface Sci. 2011, 16, 344–349. [Google Scholar] [CrossRef]
- Lim, S.; Zhang, H.; Wu, P.; Nikolov, A.; Wasan, D. The dynamic spreading of nanofluids on solid surfaces—Role of the nanofilm structural disjoining pressure. J. Colloid Interface Sci. 2016, 470, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.L.; Kondiparty, K.; Nikolov, A.D.; Wasan, D. Dynamic Spreading of Nanofluids on Solids Part II: Modeling. Langmuir 2012, 28, 16274–16284. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Choi, C.K.; Kang, Y.T.; Lee, S.H. Local aggregation characteristics of a nanofluid droplet during evaporation. Int. J. Heat Mass Transf. 2014, 72, 336–344. [Google Scholar] [CrossRef]
- Vafaei, S.; Borca-Tasciuc, T.; Podowski, M.Z.; Purkayastha, A.; Ramanath, G.; Ajayan, P.M. Effect of nanoparticles on sessile droplet contact angle. Nanotechnology 2006, 17, 2523–2527. [Google Scholar] [CrossRef]
- Munshi, A.M.; Singh, V.N.; Kumar, M.; Singh, J.P. Effect of nanoparticle size on sessile droplet contact angle. J. Appl. Phys. 2008, 103, 084315. [Google Scholar] [CrossRef]
- Vafaei, S.; Wen, D.; Borca-Tasciuc, T. Nanofluid Surface Wettability Through Asymptotic Contact Angle. Langmuir 2011, 27, 2211–2218. [Google Scholar] [CrossRef]
- Murshed, S.S.; de Castro, C.N.; Lourenco, M.; Lopes, M.; Santos, F. A review of boiling and convective heat transfer with nanofluids. Renew. Sustain. Energy Rev. 2011, 15, 2342–2354. [Google Scholar] [CrossRef]
- Lu, G.; Hu, H.; Duan, Y.; Sun, Y. Wetting kinetics of water nano-droplet containing non-surfactant nanoparticles: A molecular dynamics study. Appl. Phys. Lett. 2013, 103, 253104. [Google Scholar] [CrossRef] [Green Version]
- Estelle, P.; Cabaleiro, D.; Zyla, G.; Lugo, L.; Murshed, S.S. Current trends in surface tension and wetting behavior of nanofluids. Renew. Sustain. Energy Rev. 2018, 94, 931–944. [Google Scholar] [CrossRef]
- Chinnam, J.; Das, D.K.; Vajjha, R.S.; Satti, J.R. Measurements of the surface tension of nanofluids and development of a new correlation. Int. J. Therm. Sci. 2015, 98, 68–80. [Google Scholar] [CrossRef]
- Bhuiyan, M.; Saidur, R.; Amalina, M.; Mostafizur, R.; Islam, A. Effect of Nanoparticles Concentration and Their Sizes on Surface Tension of Nanofluids. Procedia Eng. 2015, 105, 431–437. [Google Scholar] [CrossRef] [Green Version]
- Bhuiyan, M.; Saidur, R.; Mostafizur, R.; Mahbubul, I.; Amalina, M. Experimental investigation on surface tension of metal oxide-water nanofluids. Int. Commun. Heat Mass Transf. 2015, 65, 82–88. [Google Scholar] [CrossRef]
- Shen, J.; Liburdy, J.; Pence, D.; Narayanan, V. Single Droplet Impingment: Effect of Nanoparticles. In Proceedings of the Fluids Engineering Division Summer Meeting, Jacksonville, FL, USA, 10–14 August 2008; Volume 2: Fora. [Google Scholar] [CrossRef]
- Shen, J.; Liburdy, J.A.; Pence, D.V.; Narayanan, V. Droplet impingement dynamics: Effect of surface temperature during boiling and non-boiling conditions. J. Phys. Condens. Matter 2009, 21, 464133. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Graber, C.; Liburdy, J.; Pence, D.; Narayanan, V. Simultaneous droplet impingement dynamics and heat transfer on nano-structured surfaces. Exp. Therm. Fluid Sci. 2010, 34, 496–503. [Google Scholar] [CrossRef]
- Duursma, G.; Sefiane, K.; Kennedy, A. Experimental Studies of Nanofluid Droplets in Spray Cooling. Heat Transf. Eng. 2009, 30, 1108–1120. [Google Scholar] [CrossRef]
- Murshed, S.M.S.; Nieto de Castro, C.A. Spreading Characteristics of Nanofluid Droplets Impacting onto a Solid Surface. J. Nanosci. Nanotechnol. 2011, 11, 3427–3433. [Google Scholar] [CrossRef]
- Okawa, T.; Nagano, K.; Hirano, T. Boiling heat transfer during single nanofluid drop impacts onto a hot wall. Exp. Therm. Fluid Sci. 2012, 36, 78–85. [Google Scholar] [CrossRef]
- Jackson, R.G.; Kahani, M.; Karwa, N.; Wu, A.; Lamb, R.; Taylor, R.; Rosengarten, G. Effect of surface wettability on carbon nanotube water-based nanofluid droplet impingement heat transfer. J. Phys. Conf. Ser. 2014, 525, 012024. [Google Scholar] [CrossRef] [Green Version]
- Kahani, M.; Jackson, R.G.; Rosengarten, G. Experimental Investigation of TiO2/Water Nanofluid Droplet Impingement on Nanostructured Surfaces. Ind. Eng. Chem. Res. 2016, 55, 2230–2241. [Google Scholar] [CrossRef]
- Kahani, M.; Heris, S.Z.; Mousavi, S. Comparative study between metal oxide nanopowders on thermal characteristics of nanofluid flow through helical coils. Powder Technol. 2013, 246, 82–92. [Google Scholar] [CrossRef]
- Chien, Y.C.; Weng, H.C. A Brief Note on the Magnetowetting of Magnetic Nanofluids on AAO Surfaces. Nanomaterials 2018, 8, 118. [Google Scholar] [CrossRef] [Green Version]
- Chien, Y.C.; Weng, H.C. The Effect of a Magnetic Field on the Profile of Sessile Magnetic Nanofluid Droplets. Smart Sci. 2017, 5, 214–219. [Google Scholar] [CrossRef]
- Chien, Y.C.; Weng, H.C. Magnetic Nanofluid Droplet Impact on an AAO Surface with a Magnetic Field. Appl. Sci. 2018, 8, 1059. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.L.; Shen, X.; Wang, R.; Huo, Y.; Li, C.; Wang, J. Spreading behaviors of high-viscous nanofluid droplets impact on solid surfaces. Korea-Aust. Rheol. J. 2019, 31, 167–177. [Google Scholar] [CrossRef]
- Sagawa, N. An Experimental Study of Spray Cooling in Nuclear Reactor Containers. J. Nucl. Sci. Technol. 1968, 5, 419–426. [Google Scholar] [CrossRef]
- Sawan, M.E.; Carbon, M.W. A review of spray-cooling and bottom-flooding work for lwr cores. Nucl. Eng. Des. 1975, 32, 191–207. [Google Scholar] [CrossRef]
- Breitenbach, J.; Roisman, I.V.; Tropea, C. From drop impact physics to spray cooling models: A critical review. Exp. Fluids 2018, 59, 55. [Google Scholar] [CrossRef]
- Cossali, G.E.; Coghe, A.; Marengo, M. The impact of a single drop on a wetted solid surface. Exp. Fluids 1997, 22, 463–472. [Google Scholar] [CrossRef]
- Liang, G.; Mudawar, I. Review of spray cooling—Part 1: Single-phase and nucleate boiling regimes, and critical heat flux. Int. J. Heat Mass Transf. 2017, 115, 1174–1205. [Google Scholar] [CrossRef]
- Liang, G.; Shen, S.; Guo, Y.; Zhang, J. Boiling from liquid drops impact on a heated wall. Int. J. Heat Mass Transf. 2016, 100, 48–57. [Google Scholar] [CrossRef]
- Liang, G.; Zhang, T.; Yu, H.; Chen, H.; Shen, S. Simultaneous Impact of Multiple Droplets on Liquid Film. J. Ind. Eng. Chem. 2018, 65, 51–61. [Google Scholar] [CrossRef]
- Kim, J. Spray cooling heat transfer: The state of the art. Int. J. Heat Fluid Flow 2007, 28, 753–767. [Google Scholar] [CrossRef]
- Cheng, W.L.; Zhang, W.W.; Chen, H.; Hu, L. Spray cooling and flash evaporation cooling: The current development and application. Renew. Sustain. Energy Rev. 2016, 55, 614–628. [Google Scholar] [CrossRef]
- Liu, M.S.; Lin, M.C.C.; Huang, I.T.; Wang, C.C. Enhancement of thermal conductivity with carbon nanotube for nanofluids. Int. Commun. Heat Mass Transf. 2005, 32, 1202–1210. [Google Scholar] [CrossRef]
- Assael, M.J.; Metaxa, I.N.; Kakosimos, K.; Constantinou, D. Thermal Conductivity of Nanofluids–Experimental and Theoretical. Int. J. Thermophys. 2006, 27, 999–1017. [Google Scholar] [CrossRef]
- Chopkar, M.; Das, P.K.; Manna, I. Synthesis and characterization of nanofluid for advanced heat transfer applications. Scr. Mater. 2006, 55, 549–552. [Google Scholar] [CrossRef]
- Mitra, S.; Saha, S.K.; Chakraborty, S.; Das, S. Study on boiling heat transfer of water-TiO2 and water-MWCNT nanofluids based laminar jet impingement on heated steel surface. Appl. Therm. Eng. 2012, 37, 353–359. [Google Scholar] [CrossRef]
- Hsieh, S.S.; Leu, H.Y.; Liu, H.H. Spray cooling characteristics of nanofluids for electronic power devices. Nanoscale Res. Lett. 2015, 10, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.H.; Irmawati, N. Investigation on Fluid Flow and Heat Transfer Characteristics in Spray Cooling Systems Using Nanofluids. Int. J. Aerosp. Mech. Eng. 2015, 9, 1459–1463. [Google Scholar]
- Chakraborty, S.; Sarkar, I.; Ashok, A.; Sengupta, I.; Pal, S.K.; Chakraborty, S. Synthesis of Cu-Al LDH nanofluid and its application in spray cooling heat transfer of a hot steel plate. Powder Technol. 2018, 335, 285–300. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sarkar, I.; Ashok, A.; Sengupta, I.; Pal, S.K.; Chakraborty, S. Thermo-physical properties of Cu-Zn-Al LDH nanofluid and its application in spray cooling. Appl. Therm. Eng. 2018, 141, 339–351. [Google Scholar] [CrossRef]
- Bellerova, H.; Tseng, A.A.; Pohanka, M.; Raudensky, M. Spray cooling by solid jet nozzles using alumina/water nanofluids. Int. J. Therm. Sci. 2012, 62, 127–137. [Google Scholar] [CrossRef]
- Bellerova, H.; Tseng, A.A.; Pohanka, M.; Raudensky, M. Heat transfer of spray cooling using alumina/water nanofluids with full cone nozzles. Heat Mass Transf. 2012, 48, 1971–1983. [Google Scholar] [CrossRef]
- Tseng, A.A.; Bellerova, H.; Pohanka, M.; Raudensky, M. Effects of Titania nanoparticles on heat transfer performance of spray cooling with full cone nozzle. Appl. Therm. Eng. 2014, 62, 20–27. [Google Scholar] [CrossRef]
- Chang, T.B.; Syu, S.C.; Yang, Y.K. Effects of particle volume fraction on spray heat transfer performance of Al2O3-water nanofluid. Int. J. Heat Mass Transf. 2012, 55, 1014–1021. [Google Scholar] [CrossRef]
- Maly, M.; Moita, A.S.; Jedelsky, J.; Ribeiro, A.P.C.; Moreira, A.L.N. Effect of nanoparticles concentration on the characteristics of nanofluid sprays for cooling applications. J. Therm. Anal. Calorim. 2019, 135, 3375–3386. [Google Scholar] [CrossRef] [Green Version]
- Kang, B.; Marengo, M.; Begg, S. A Study of the Effect of Nanoparticle Concentration on the Characteristics of Nanofluid Sprays. J. Appl. Fluid Mech. 2019, 12, 413–420. [Google Scholar] [CrossRef]
- Wang, B.X.; Zhou, L.P.; Peng, X.F. A fractal model for predicting the effective thermal conductivity of liquid with suspension of nanoparticles. Int. J. Heat Mass Transf. 2003, 46, 2665–2672. [Google Scholar] [CrossRef]
- Evans, W.; Prasher, R.; Fish, J.; Meakin, P.; Phelan, P.; Keblinski, P. Effect of aggregation and interfacial thermal resistance on thermal conductivity of nanocomposites and colloidal nanofluids. Int. J. Heat Mass Transf. 2008, 51, 1431–1438. [Google Scholar] [CrossRef]














| Reference | Fluid | [mm] | [mN/m] | Impingement Conditions | Recording Properties | Surface | Remarks |
|---|---|---|---|---|---|---|---|
| Shen et al. [153] | Water + ...Au (5 nm) ...Au (10 nm) | 1.85 1.27 1.24 | 72.34 72.36 69.91 | m/s | 16,000 fps 9 µm/px | Heated silicon |
|
| Shen et al. [154] | SWCNT + Water | 1.29 | 70.52 | Re = 1700 | 7400 fps |
|
|
| 1.18 | 72.69 | We = 25 |
| ||||
| Duursma et al. [156] | AlO + (20–50 nm) ...Water ...Ethanol ...DMSO | We = 44–183 | Insulated copper block |
| |||
| Murshed et al. [157] | TiO + EG (15 nm) | 1.4 | 42.3 | We = 53–106 | 500 fps | Aluminum (22–170C) |
|
| Okawa et al. [158] | TiO + water (21 nm) | 3.3 | We = 25–239 | 2000 fps | SS 304 plate |
| |
| Jackson et al. [159] | DWCN + water (3.5 nm dia., 1–10 m length) | ≈ 39 | 8700 fps (870 fps) IR camera | Monocrystalline silicon wafer (40 C)
|
| ||
| Kahani et al. [160] | TiO + water (21 nm) | 1.57 | 8700 fps (870 fps) IR camera | Silicon wafer
|
| ||
| Chien et al. [164] | FeO + water | ≈ 3.4 (0.02 mL) | 2000 fps | Anodic Aluminum Oxide (AAO) |
| ||
| Liu et al. [165] | MWCNT + (Nano-graphite) (Nano-graphene) | 2.45 | 52–55 | We = 50–800 | 10,000 fps | Glass (Hydrophilic) Teflon (Hydrophobic) |
|
| Epoxy resin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aksoy, Y.T.; Zhu, Y.; Eneren, P.; Koos, E.; Vetrano, M.R. The Impact of Nanofluids on Droplet/Spray Cooling of a Heated Surface: A Critical Review. Energies 2021, 14, 80. https://doi.org/10.3390/en14010080
Aksoy YT, Zhu Y, Eneren P, Koos E, Vetrano MR. The Impact of Nanofluids on Droplet/Spray Cooling of a Heated Surface: A Critical Review. Energies. 2021; 14(1):80. https://doi.org/10.3390/en14010080
Chicago/Turabian StyleAksoy, Yunus Tansu, Yanshen Zhu, Pinar Eneren, Erin Koos, and Maria Rosaria Vetrano. 2021. "The Impact of Nanofluids on Droplet/Spray Cooling of a Heated Surface: A Critical Review" Energies 14, no. 1: 80. https://doi.org/10.3390/en14010080
APA StyleAksoy, Y. T., Zhu, Y., Eneren, P., Koos, E., & Vetrano, M. R. (2021). The Impact of Nanofluids on Droplet/Spray Cooling of a Heated Surface: A Critical Review. Energies, 14(1), 80. https://doi.org/10.3390/en14010080