A New Method of Regulating the Cooling Capacity of a Cooling System with CO2
Abstract
:1. Introduction
1.1. Carbon Dioxide as a Refrigerant
1.2. Methods of Regulating Cooling Capacity and Their Influence on EER
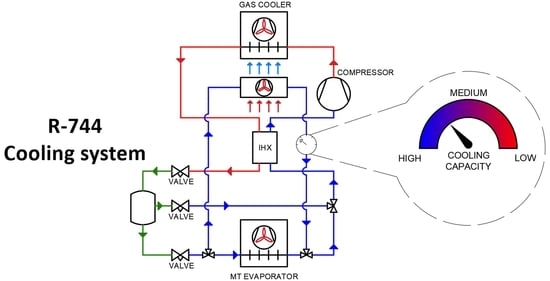
2. System Description
2.1. Installation with a Hot Gas Bypass Valve
2.2. Installation with an Additional Evaporator—Air Cooler
3. Computational Model
3.1. Initial Conditions
3.2. Energy Equations for a Cooling Capacity Regulation Method with the Additional Evaporator
3.3. Energy Equations for a Cooling Capacity Regulation Method with the Hot Gas Bypass Valve
4. Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| a | Ratio of Qi to Q0 |
| COP | Coefficient of performance |
| cp | Specific heat capacity of air, J/(kgK) |
| EER | Energy efficiency ratio |
| EERi | Reduced energy efficiency ratio |
| EERN | Nominal energy efficiency ratio |
| h1 | Enthalpy of the refrigerant before the compressor, J/kg |
| h10 | Enthalpy of the refrigerant before the compressor during cooling regulation with a compressor bypass valve, J/kg |
| h2 | Enthalpy of the refrigerant after the compressor, J/kg |
| h2,i | Enthalpy of the refrigerant after the compressor during cooling regulation, J/kg |
| h2s | Isentropic compression enthalpy of the refrigerant, J/kg |
| h6 | Enthalpy of the refrigerant before the medium-temperature evaporator, J/kg |
| h7″ | Enthalpy of the refrigerant after the medium-temperature evaporator, J/kg |
| HP | High pressure |
| IHX | Internal heat exchanger |
| IP | Intermediate pressure |
| MT | Medium temperature |
| Dry vapor refrigerant mass flow rate, kg/s | |
| Mass flow rate of the refrigerant in the evaporator, kg/s | |
| Mass flow rate of the refrigerant proportional to the reduction of the cooling load, kg/s | |
| Air mass flow rate, kg/s | |
| Total mass flow rate of the refrigerant, kg/s | |
| Nc | Compressor power, W |
| Nc,i | Reduced compressor power, W |
| pIP | Intermediate pressure, bar |
| pk | High pressure, bar |
| popt | Optimal high pressure, bar |
| p0 | Evaporating pressure, bar |
| qIHX | Unit heat flow in the internal heat exchanger, kJ/kg |
| Q0 | Nominal cooling capacity, W |
| Qi | Cooling capacity decrease, W |
| Rp | Pressure ratio |
| tair | Ambient air temperature, °C |
| tair,in | Inlet ambient air temperature, °C |
| tair,out | Outlet ambient air temperature, °C |
| t0 | Evaporating temperature, °C |
| x | Vapor quality |
| ΔEER | Relative change in EER |
| ΔN | Relative change in compressor power |
| ηis | Isentropic efficiency |
References
- WHO. The Montreal Protocol on Substances that Deplete the Ozone Layer; WHO: Montreal, QC, Canada, 1987. [Google Scholar]
- FCCC. The Kyoto Protocol to the United Nations Framework Convention on Climate Change; FCCC: Kyoto, Japan, 1997.
- Wang, R.Z.; Li, Y. Perspectives for natural working fluids in China. Int. J. Refrig. 2007, 30, 568–581. [Google Scholar] [CrossRef]
- Zheng, G.; Jing, Y. Air Conditioning: Selecting the Optimal Cool Storage System. Energy Environ. 2007, 18, 251–257. [Google Scholar] [CrossRef]
- Pearson, A. Carbon dioxide—New uses for an old refrigerant. Int. J. Refrig. 2005, 28, 1140–1148. [Google Scholar] [CrossRef]
- ISO. ISO 817:2014. Refrigerants—Designation and Safety Classification; ISO: Geneva, Switzerland, 2014. [Google Scholar]
- Bieniek, A.; Pysz, M.; Mika, Ł. Alternatywne czynniki chłodnicze R-1234yf oraz R-744 stosowane w samochodowej instalacji klimatyzacyjnejod 2017 roku. Zesz. Nauk. Inst. Gospod. Surowcami Miner. Energią Pol. Akad. Nauk 2018, 103, 117–132. [Google Scholar] [CrossRef]
- Sineviciene, L.; Sotnyk, I.; Kubatko, O. Determinants of energy efficiency and energy consumption of Eastern Europe post-communist economies. Energy Environ. 2017, 28, 870–884. [Google Scholar] [CrossRef]
- Sarkar, J.; Bhattacharyya, S.; Gopal, M.R. Optimization of a transcritical CO2 heat pump cycle for simultaneous cooling and heating applications. Int. J. Refrig. 2004, 27, 830–838. [Google Scholar] [CrossRef]
- Dai, B.; Liu, S.; Li, H.; Sun, Z.; Song, M.; Yang, Q.; Ma, Y. Energetic performance of transcritical CO2 refrigeration cycles with mechanical subcooling using zeotropic mixture as refrigerant. Energy 2018, 150, 205–221. [Google Scholar] [CrossRef]
- Sharma, V.; Fricke, B.; Bansal, P. Comparative analysis of various CO2 configurations in supermarket refrigeration systems. Int. J. Refrig. 2014, 46, 86–99. [Google Scholar] [CrossRef]
- Srinivasan, K.; Sheahen, P.; Sarathy, C.S.P. Optimum thermodynamic conditions for upper pressure limits of transcritical carbon dioxide refrigeration cycle. Int. J. Refrig. 2010, 33, 1395–1401. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Z.; Tian, H. A review of transcritical carbon dioxide heat pump and refrigeration cycles. Energy 2013, 55, 156–172. [Google Scholar] [CrossRef]
- Ge, Y.T.; Tassou, S.A. Performance evaluation and optimal design of supermarket refrigeration systems with supermarket model “superSim”. Part II: Model applications. Int. J. Refrig. 2011, 34, 540–549. [Google Scholar] [CrossRef] [Green Version]
- Belusko, M.; Liddle, R.; Alemu, A.; Halawa, E.; Bruno, F. Performance evaluation of a CO2 refrigeration system enhanced with a dew point cooler. Energies 2019, 12, 1079. [Google Scholar] [CrossRef] [Green Version]
- Bruno, F.; Belusko, M.; Halawa, E. CO2 Refrigeration and Heat Pump Systems—A Comprehensive Review. Energies 2019, 12, 2959. [Google Scholar] [CrossRef] [Green Version]
- Gullo, P. Advanced thermodynamic analysis of a transcritical R744 booster refrigerating unit with dedicated mechanical subcooling. Energies 2018, 11, 3058. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, D.; Catalán-Gil, J.; Cabello, R.; Calleja-Anta, D.; Llopis, R.; Nebot-Andrés, L. Experimental analysis and optimization of an R744 transcritical cycle working with a mechanical subcooling system. Energies 2020, 13, 3204. [Google Scholar] [CrossRef]
- Gullo, P.; Hafner, A.; Banasiak, K. Thermodynamic performance investigation of commercial R744 booster refrigeration plants based on advanced exergy analysis. Energies 2019, 12, 354. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Sun, Y.; Tang, D. Analysis of a CO2 transcritical refrigeration cycle with a vortex tube expansion. Sustainability 2019, 11, 2021. [Google Scholar] [CrossRef] [Green Version]
- Bellos, E.; Tzivanidis, C. A Theoretical Comparative Study of CO2 Cascade Refrigeration Systems. Appl. Sci. 2019, 9, 790. [Google Scholar] [CrossRef] [Green Version]
- Lorentzen, G. Revival of carbon dioxide as a refrigerant. Int. J. Refrig. 1994, 17, 292–301. [Google Scholar] [CrossRef]
- Purohit, N.; Sharma, V.; Sawalha, S.; Fricke, B.; Llopis, R.; Dasgupta, M.S. Integrated supermarket refrigeration for very high ambient temperature. Energy 2018, 165, 572–590. [Google Scholar] [CrossRef]
- Girotto, S.; Minetto, S.; Neksa, P. Commercial refrigeration system using CO2 as the refrigerant. Int. J. Refrig. 2004, 27, 717–723. [Google Scholar] [CrossRef]
- Gullo, P.; Hafner, A.; Banasiak, K. Transcritical R744 refrigeration systems for supermarket applications: Current status and future perspectives. Int. J. Refrig. 2018, 93, 269–310. [Google Scholar] [CrossRef]
- Haida, M.; Smolka, J.; Hafner, A.; Ostrowski, Z.; Palacz, M.; Madsen, K.B.; Försterling, S.; Nowak, A.J.; Banasiak, K. Performance mapping of the R744 ejectors for refrigeration and air conditioning supermarket application: A hybrid reduced-order model. Energy 2018, 153, 933–948. [Google Scholar] [CrossRef]
- Rony, R.; Yang, H.; Krishnan, S.; Song, J. Recent Advances in Transcritical CO2 (R744) Heat Pump System: A Review. Energies 2019, 12, 457. [Google Scholar] [CrossRef] [Green Version]
- Cabello, R.; Sánchez, D.; Llopis, R.; Torrella, E. Experimental evaluation of the energy efficiency of a CO2 refrigerating plant working in transcritical conditions. Appl. Therm. Eng. 2008, 28, 1596–1604. [Google Scholar] [CrossRef]
- Chesi, A.; Esposito, F.; Ferrara, G.; Ferrari, L. Experimental analysis of R744 parallel compression cycle. Appl. Energy 2014, 135, 274–285. [Google Scholar] [CrossRef]
- Sarkar, J. Optimization of ejector-expansion transcritical CO2 heat pump cycle. Energy 2008, 33, 1399–1406. [Google Scholar] [CrossRef]
- Llopis, R.; Cabello, R.; Sánchez, D.; Torrella, E. Energy improvements of CO2 transcritical refrigeration cycles using dedicated mechanical subcooling. Int. J. Refrig. 2015, 55, 129–141. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.L.; Ma, Y.T.; Liu, S.C. Performance investigation of transcritical carbon dioxide two-stage compression cycle with expander. Energy 2007, 32, 237–245. [Google Scholar] [CrossRef]
- Bellos, E.; Tzivanidis, C. A comparative study of CO2 refrigeration systems. Energy Convers. Manag. X 2019, 1, 100002. [Google Scholar] [CrossRef]
- Torrella, E.; Sánchez, D.; Llopis, R.; Cabello, R. Energetic evaluation of an internal heat exchanger in a CO2 transcritical refrigeration plant using experimental data. Int. J. Refrig. 2011, 34, 40–49. [Google Scholar] [CrossRef]
- Borowski, P.F. Adaptation strategy on regulated markets of power companies in Poland. Energy Environ. 2019, 30, 3–26. [Google Scholar] [CrossRef]
- Krzywanski, J.; Sztekler, K.; Szubel, M.; Siwek, T.; Nowak, W.; Mika, Ł. A comprehensive three-dimensional analysis of a large-scale multi-fuel CFB boiler burning coal and syngas. Part 1. The CFD model of a large-scale multi-fuel CFB combustion. Entropy 2020, 22, 964. [Google Scholar] [CrossRef]
- Krzywanski, J.; Sztekler, K.; Szubel, M.; Siwek, T.; Nowak, W.; Mika, L. A comprehensive, three-dimensional analysis of a large-scale, multi-fuel, CFB boiler burning coal and syngas. Part 2. Numerical simulations of coal and syngas co-combustion. Entropy 2020, 22, 856. [Google Scholar] [CrossRef] [PubMed]
- Sztekler, K.; Kalawa, W.; Mika, L.; Krzywanski, J.; Grabowska, K.; Sosnowski, M.; Nowak, W.; Siwek, T.; Bieniek, A. Modeling of a combined cycle gas turbine integrated with an adsorption chiller. Energies 2020, 13, 515. [Google Scholar] [CrossRef] [Green Version]
- Bell, I.H.; Wronski, J.; Quoilin, S.; Lemort, V. Pure and pseudo-pure fluid thermophysical property evaluation and the open-source thermophysical property library coolprop. Ind. Eng. Chem. Res. 2014, 53, 2498–2508. [Google Scholar] [CrossRef] [Green Version]










| Cycle | tair, (°C) | pk, (bar) | tref, out, (°C) | p0, (bar) | t0, (°C) | pIP, (bar) | Q0, (kW) | m1 (kg/s) | m0, (kg/s) | qIHX (kJ/kg) |
|---|---|---|---|---|---|---|---|---|---|---|
| Subcritical | 15 | 64.62 | 20 | 30 | −5 | 45 | 10 | 0.043 | 0 | 26.6 |
| Supercritical | 35 | 93.5 | 40 | 30 | −5 | 45 | 10 | 0.048 | 0.03 | 26.6 |
| Point | p, (bar) | t, (°C) | x, (-) |
|---|---|---|---|
| 1 | 30.0 | 10.0 | − |
| 2s | 64.6 | 69.0 | − |
| 2 | 64.6 | 79.5 | − |
| 3 | 64.6 | 20.0 | − |
| 4 | 64.6 | 11.0 | − |
| 5 | 45.0 | 9.5 | − |
| 6 | 30.0 | −5.0 | 0.15 |
| 7″ | 30.0 | −5.0 | 1.00 |
| Point | p, (bar) | t, (°C) | x, (-) |
|---|---|---|---|
| 1 | 30.0 | 10.0 | − |
| 2s | 93.5 | 100.6 | − |
| 2 | 93.5 | 121.2 | − |
| 3 | 93.5 | 40.0 | − |
| 4 | 93.5 | 14.6 | − |
| 5 | 45.0 | 10.0 | 0.38 |
| 5′ | 45.0 | 10.0 | 0 |
| 5″ | 45.0 | 10.0 | 1.00 |
| 6 | 30.0 | −5.0 | 0.16 |
| 7″ | 30.0 | −5.0 | 1.00 |
| 8 | 30.0 | −5.0 | 0.96 |
| 9 | 30.0 | −5.0 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bieniek, A.; Kuchmacz, J.; Sztekler, K.; Mika, L.; Radomska, E. A New Method of Regulating the Cooling Capacity of a Cooling System with CO2. Energies 2021, 14, 1922. https://doi.org/10.3390/en14071922
Bieniek A, Kuchmacz J, Sztekler K, Mika L, Radomska E. A New Method of Regulating the Cooling Capacity of a Cooling System with CO2. Energies. 2021; 14(7):1922. https://doi.org/10.3390/en14071922
Chicago/Turabian StyleBieniek, Artur, Jan Kuchmacz, Karol Sztekler, Lukasz Mika, and Ewelina Radomska. 2021. "A New Method of Regulating the Cooling Capacity of a Cooling System with CO2" Energies 14, no. 7: 1922. https://doi.org/10.3390/en14071922
APA StyleBieniek, A., Kuchmacz, J., Sztekler, K., Mika, L., & Radomska, E. (2021). A New Method of Regulating the Cooling Capacity of a Cooling System with CO2. Energies, 14(7), 1922. https://doi.org/10.3390/en14071922