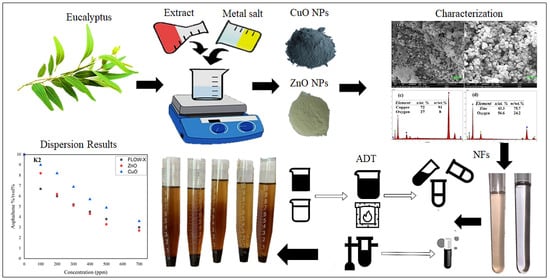
Efficacy of Green Oxide Nanofluids as Potential Dispersants for Asphaltene in Iraqi Crudes, Experimental, Tunning and Statistical Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Eucalyptus Leaf Extract
2.2. Synthesis of Green CuO and ZnO Nanoparticles
2.3. Syntheses of CuO and ZnO Nonfluids/Chemical
2.4. Asphaltene Dispersion Test
Asphaltene Dispersion Test ADT
2.5. Extraction of Asphaltene
3. Results
3.1. UV–Vis of the Plant Extract
3.2. FE-SEM, EDX, and Elemental Mapping Analysis
3.3. X-ray Diffraction (XRD)
3.4. Nanofluid Stability
3.5. Asphaltene Dispersion Test
3.6. Blank Test Results
3.7. ADT Results at 100–700 ppm Doses of Cuo, ZnO, and FLOW-X
3.8. Asphaltene Dispersion from UV–Vis Tests
3.9. Inhibitor Efficacy and Feasibility of This Research
4. Discussion
- Control tests for ADT were kept at 10% asphaltene for all samples, which can give precise measurements and a good baseline indicator to compare the results of each inhibitor of this research to each other.
- ADT is a simple and well-documented test and can be used in oil field trials to monitor inhibitor performance.
- At the 100 ppm dosage, the controlling factor was crude oil type in terms of API, Specific gravity, and asphaltene content.
- A 500 ppm dosage of ZnO gave the best result out of the three inhibitors used, while CuO for K7 was very efficient.
- At 100 ppm, FLOW-X was better in inhibiting asphaltene as it is used in oil fields.
- At 700 ppm, there was a negligible improvement, especially for CuO and ZnO NPs, and the advantages of lower ppm outweigh the added cost of increasing the concentration.
5. Conclusions
- The ZnO nanofluid/chemical was the most efficient in terms of concentration per asphaltene dispersion.
- The CuO nanofluid/chemical had the efficiency dispersion percentage of asphaltene at heavy crudes.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thawer, R.; Nicoll, D.C.A.; Dick, G. Asphaltene Deposition in Production Facilities. SPE Prod. Eng. 1990, 5, 475–480. [Google Scholar] [CrossRef]
- Rivero-Sanchez, J.A.; Ramos-Pallares, F.; Schoeggl, F.F.; Yarranton, H.W. Asphaltene Precipitation from Heavy Oil Diluted with Petroleum Solvents. Energy Fuels 2021, 35, 9396–9407. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Kharrat, R. A Compositional Reservoir Simulation and Experimental Investigation of Asphaltene Onset Pressure. Pet. Sci. Technol. 2014, 32, 2253–2262. [Google Scholar] [CrossRef]
- Soleymanzadeh, A.; Yousefi, M.; Kord, S.; Mohammadzadeh, O. A review on methods of determining onset of asphaltene precipitation. Journal of Petroleum Explor. Prod. Technol. 2019, 9, 1375–1396. [Google Scholar] [CrossRef]
- Doryani, H.; Malayeri, M.R.; Riazi, M. Visualization of asphaltene precipitation and deposition in a uniformly patterned glass micromodel. Fuel 2016, 182, 613–622. [Google Scholar] [CrossRef]
- Madhi, M.; Kharrat, R.; Hamoule, T. Screening of inhibitors for remediation of asphaltene deposits: Experimental and modeling study. Petroleum 2017, 4. [Google Scholar] [CrossRef]
- Mohammadi, M.; Dadvar, M.; Dabir, B. TiO2/SiO2 nanofluids as novel inhibitors for the stability of asphaltene particles in crude oil: Mechanistic understanding, screening, modeling, and optimization. J. Mol. Liq. 2017, 238, 326–340. [Google Scholar] [CrossRef]
- Hoepfner, M. Investigations into Asphaltene Deposition, Stability, and Structure. 2013. Available online: https://hdl.handle.net/2027.42/100081 (accessed on 15 July 2022).
- Ahmadi, Y.; Eshraghi, S.E.; Bahrami, P.; Hasanbeygi, M.; Kazemzadeh, Y.; Vahedian, A. Comprehensive Water–Alternating-Gas (WAG) injection study to evaluate the most effective method based on heavy oil recovery and asphaltene precipitation tests. J. Pet. Sci. Eng. 2015, 133, 123–129. [Google Scholar] [CrossRef]
- Kazemzadeh, Y.; Eshraghi, S.E.; Kazemi, K.; Sourani, S.; Mehrabi, M.; Ahmadi, Y. Behavior of Asphaltene Adsorption onto the Metal Oxide Nanoparticle Surface and Its Effect on Heavy Oil Recovery. Ind. Eng. Chem. Res. 2015, 54, 233–239. [Google Scholar] [CrossRef]
- Liu, H.; Jin, X.; Ding, B. Application of nanotechnology in petroleum exploration and development. Pet. Explor. Dev. 2016, 43, 1107–1115. [Google Scholar] [CrossRef]
- Ahmadi, Y. Relationship between Asphaltene Adsorption on the Surface of Nanoparticles and Asphaltene Precipitation Inhibition During Real Crude Oil Natural Depletion Tests. Iran. J. Oil Gas Sci. Technol. 2021, 10, 69–82. [Google Scholar]
- Ahmadi, Y.; Aminshahidy, B. Effects of hydrophobic CaO and SiO2 nanoparticles on Asphaltene Precipitation Envelope (APE): An experimental and modeling approach. Oil Gas Sci. Technol. 2018, 73, 56. [Google Scholar] [CrossRef]
- Alhreez, M.; Wen, D.; Ali, L. A novel inhibitor for controlling Iraqi asphaltene problems. In Proceedings of the 2017 International Conference on Environmental Impacts of the Oil and Gas Industries: Kurdistan Region of Iraq as a Case Study (EIOGI), Koya-Erbil, Iraq, 17–19 April 2017. [Google Scholar]
- Mohammadi, M.; Akbari, M.; Fakhroueian, Z.; Bahramian, A.; Azin, R.; Arya, S. Inhibition of Asphaltene Precipitation by TiO2, SiO2, and ZrO2 Nanofluids. Energy Fuels 2011, 25, 3150–3156. [Google Scholar] [CrossRef]
- Ahmadbaygi, A.; Bayati, B.; Mansouri, M.; Rezaei, H.; Riazi, M. Chemical study of asphaltene inhibitors effects on asphaltene precipitation of an Iranian oil field. Oil Gas Sci. Technol. 2020, 75, 6. [Google Scholar] [CrossRef]
- Aguiar, J.I.S.; Punase, A.; Mazzeo, C. Influence of Asphaltene Inhibitors on Wax and Asphaltene Deposition—Are Problems Associated? In Proceedings of the Offshore Technology Conference Brasil, Rio de Janeiro, Brazil, 29–31 October 2019. [Google Scholar]
- Mandal, A.; Deka, B.; Mahto, V.; Nihalani, M.C.; Purohit, S. Synthesis, characterization and evaluation of a novel asphaltene inhibitor to control organic solid deposition in petroleum formation. Pet. Sci. Technol. 2019, 37, 780–786. [Google Scholar] [CrossRef]
- Chen, C.; Guo, J.; An, N.; Pan, Y.; Li, Y.; Jiang, Q. Study of asphaltene dispersion and removal for high-asphaltene oil wells. Pet. Sci. 2012, 9, 551–557. [Google Scholar] [CrossRef]
- Alemi, F.M.; Mohammadi, S.; Dehghani, S.A.M.; Rashidi, A.; Hosseinpour, N.; Seif, A. Experimental and DFT studies on the effect of carbon nanoparticles on asphaltene precipitation and aggregation phenomena. Chem. Eng. J. 2021, 422, 130030. [Google Scholar] [CrossRef]
- Manek, M.B. Asphaltene Dispersants as Demulsification Aids. In Proceedings of the SPE International Symposium on Oilfield Chemistry, San Antonio, TX, USA, 14–17 February 1995. [Google Scholar]
- Sayyad Amin, J.; Nikkhah, S.; Zendehboudi, S. A new experimental and modeling strategy to determine asphaltene precipitation in crude oil. Chem. Eng. Res. Des. 2017, 128, 162–173. [Google Scholar] [CrossRef]
- Mertz, C.; Cheynier, V.; Günata, Z.; Brat, P. Analysis of Phenolic Compounds in Two Blackberry Species (Rubus glaucus and Rubus adenotrichus) by High-Performance Liquid Chromatography with Diode Array Detection and Electrospray Ion Trap Mass Spectrometry. J. Agric. Food Chem. 2007, 55, 8616–8624. [Google Scholar] [CrossRef]
- Shiraishi, M.; Inagaki, M. Chapter 10—X-ray Diffraction Methods to Study Crystallite Size and Lattice Constants of Carbon Materials. In Carbon Alloys; Yasuda, E.-I., Ingaki, M., Kaneko, K., Endo, M., Oya, A., Tanabe, Y., Eds.; Elsevier Science: Oxford, UK, 2003; pp. 161–173. [Google Scholar]
- Saleh, T.A. Chapter 7—Structural characterization of hybrid materials. In Polymer Hybrid Materials and Nanocomposites; Saleh, T.A., Ed.; William Andrew Publishing: Norwich, NY, USA, 2021; pp. 213–240. [Google Scholar]
- Ahmadi, Y.; Aminshahidy, B. Inhibition of asphaltene precipitation by hydrophobic CaO and SiO2 nanoparticles during natural depletion and CO2 tests. Int. J. Oil Gas Coal Technol. 2020, 24, 394. [Google Scholar] [CrossRef]
- Mansouri, M.; Ahmadi, Y.; Jafarbeigi, E. Introducing a new method of using nanocomposites for preventing asphaltene aggregation during real static and dynamic natural depletion tests. Energy Sources Part A Recovery Util. Environ. Eff. 2022, 44, 7499–7513. [Google Scholar] [CrossRef]











| Author | year | SARA% | API | Inhibitor | AOS | Outcome |
|---|---|---|---|---|---|---|
| [7] | 2017 | Asp 8.2%−11.4 | 22.4 26.7 | 0.04 wt% (80%TiO2:20 %SiO2 | production | The onset of asphaltene flocculation (the n-heptane volume increased). |
| [13] | 2018 | Saturate% 56.2 Aromatic% 34.1 Resin% 7.7 Asphaltene% 2.0 | 37 | CaO 45 ppm SiO2 45 ppm | production/EOR | In wide ranges of data, as temperature increased, asphaltene upper onset pressure increased. CaO and SiO2 nanoparticles decreased asphaltene precipitations in the presence of CO. |
| [14] | 2017 | (Poly (vinyltoluene-co-alpha-methylstyrene)) 6% wt | production | Asphaltene inhibitor makes the asphaltene particles more stable due to its unique ability to interact between asphaltene particles and asphaltene inhibitor molecules via p–p interactions and hydrogen bonding. | ||
| [15] | 2011 | Asph 10% wt | 19 | TiO2 + HNO3 (65%) | production | Results show that rutile (TiO2) fine nanoparticles can effectively enhance the asphaltene stability in acidic conditions and act inversely in basic conditions. |
| [16] | 2020 | A, B, C 500 ppm | production | The results show that when an asphaltene inhibitor is not injected into the mixture of synthetic oil/n-heptane, AOP (Asphaltene Onset Point) occurs at 35 vol.% of n-heptane, while with addition of 3000 ppm of asphaltene B inhibitor, AOP occurs at 60 vol.% of n-heptane. | ||
| [17] | 2019 | 45.6 41 7.6 5.8 | 31.7 | AI 1, 500 ppm | production | The efficiency of the AI on dispersing the asphaltenes was observed to have a major impact on the precipitation as possibly increasing the deposition of microcrystalline waxes. |
| [18] | 2019 | 45 16.5 8.5 3 Wax 12 | 25.03 | n-phenylamino hexanol | production | Asphaltene deposition in the pores of sandstone core is studied by flooding the virgin and additive beneficiated crude oil indicating less deposition in beneficiated crude oil. |
| [19] | 2012 | 28.04 21.16 12.48 38.32 | SDJ agent 1% wt | production | Colloid instability index greater than 0.9 can effectively inhibit asphaltene deposition in the wellbore. | |
| [20] | 2021 | CNPs | reservoir | Aggregation of asphaltene can be delayed from 26 to 37% Vol n-C7 with the existence of 400 ppm CNPs. |
| Sample | Sample Point | Sp, Gr | API | Grade | Known Asphaltene Problem |
|---|---|---|---|---|---|
| K1 | main stream | 0.916 | 23 | Medium | Non |
| K2 | well head | 0.904 | 25 | Medium | Moderate |
| K3 | well head | 0.86 | 33 | Light | Non |
| K4 | well head | 0.871 | 31 | light | Moderate |
| K5 | wellhead | 0.898 | 26 | Medium | Non |
| K6 | separator | 0.84 | 37 | Light | Non |
| K7 | wellhead | 0.993 | 11 | U. Heavy | Sever |
| K8 | storage tank | 0.887 | 28 | Intermediate | Non |
| K9 | mainstream | 0.882 | 29 | Intermediate | Moderate |
| Name | Particle% | Xylene% | DCM% | DBSA% | Toluene% | Benzene% |
|---|---|---|---|---|---|---|
| ZnO NF | <1% | 15% | 40% | 2% | 17% | 25% |
| CuO NF | <1% | 15% | 40% | - | 19% | 25% |
| Peak Position 2θ (°) | FWHM Bsize (°) | D-Spacing (Å) | Dp (nm) |
|---|---|---|---|
| 31.2316 | 0.048 | 2.86159 | 179.6245 |
| 31.3156 | 0.036 | 2.86120 | 239.5484 |
| 31.7262 | 0.288 | 2.81810 | 29.97385 |
| 35.6244 | 0.288 | 2.51816 | 30.28406 |
| 38.863 | 0.336 | 2.31544 | 26.20617 |
| 45.4286 | 0.288 | 1.99489 | 31.25658 |
| 48.8144 | 0.384 | 1.86414 | 23.74646 |
| 53.4745 | 1.152 | 1.71216 | 8.071059 |
| 58.3345 | 0.768 | 1.58055 | 12.38219 |
| 61.625 | 0.768 | 1.50381 | 12.58912 |
| 66.174 | 0.768 | 1.41103 | 12.90474 |
| 68.1274 | 0.48 | 1.37525 | 20.88255 |
| Average FWHM | 0.467 | Average size | 52.2 |
| Peak Position 2θ (°) | FWHM Bsize (°) | D-Spacing (Å) | Dp (nm) |
|---|---|---|---|
| 31.7811 | 0.2755 | 2.81569 | 31.34 |
| 34.4442 | 0.551 | 2.60384 | 15.78 |
| 36.3632 | 0.4723 | 2.47072 | 18.51 |
| 47.5726 | 0.4723 | 1.91145 | 19.21 |
| 56.4683 | 0.4723 | 1.62963 | 19.96 |
| 62.8988 | 0.7872 | 1.47761 | 12.36 |
| Average FWHM | 0.5051 | Average size | 19.52588 |
| Sample | 400/Dis FLOW-X | 400/Dis ZnO | 400/Dis CuO |
|---|---|---|---|
| K1 | 7.8 | 7.2 | 9.3 |
| K2 | 7.3 | 7.0 | 9.3 |
| K3 | 6.1 | 5.9 | 8.4 |
| K4 | 5.9 | 5.9 | 7.8 |
| K5 | 6.3 | 6.3 | 8.9 |
| K6 | 5.4 | 5.1 | 6.9 |
| K7 | 10.0 | 8.2 | 8.0 |
| K8 | 6.0 | 6.8 | 7.8 |
| K9 | 6.9 | 6.3 | 6.8 |
| Average | 6.8 | 6.5 | 8.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khidhir, D.; Sidiq, H. Efficacy of Green Oxide Nanofluids as Potential Dispersants for Asphaltene in Iraqi Crudes, Experimental, Tunning and Statistical Analysis. Energies 2022, 15, 6833. https://doi.org/10.3390/en15186833
Khidhir D, Sidiq H. Efficacy of Green Oxide Nanofluids as Potential Dispersants for Asphaltene in Iraqi Crudes, Experimental, Tunning and Statistical Analysis. Energies. 2022; 15(18):6833. https://doi.org/10.3390/en15186833
Chicago/Turabian StyleKhidhir, Dana, and Hiwa Sidiq. 2022. "Efficacy of Green Oxide Nanofluids as Potential Dispersants for Asphaltene in Iraqi Crudes, Experimental, Tunning and Statistical Analysis" Energies 15, no. 18: 6833. https://doi.org/10.3390/en15186833
APA StyleKhidhir, D., & Sidiq, H. (2022). Efficacy of Green Oxide Nanofluids as Potential Dispersants for Asphaltene in Iraqi Crudes, Experimental, Tunning and Statistical Analysis. Energies, 15(18), 6833. https://doi.org/10.3390/en15186833