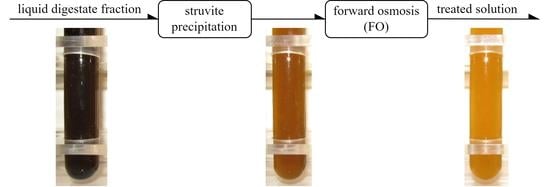
Treatment of Liquid Fraction of Digestate by Integrated Process Struvite Precipitation—Forward Osmosis
Abstract
:1. Introduction
- gases dissolved in water (SO2 or a mixture of NH3 and CO2)
- sugars (glucose, fructose, sucrose)
- inorganic salts (NaCl, MgCl2, CaCl2, Al2(SO4)3)
- organic salts (Na+ and Mg2+ salts of formic, acetic or propionic acid)
- hydrophilic magnetic nanoparticles.
2. Materials and Methods
3. Results
4. Conclusions
- Struvite precipitation allowed for organic compounds removal of 2.5–9.5% and removal of Mg/Ca/P up to ca. 90%.
- A novel concept of struvite precipitation from liquid digestate applied at the solution pretreatment stage before FO allowed us to obtain a final solution of significant quality (DOC removed by 99.9%) and protected the membranes from excessive blockage.
- FO performed on a stream of digestate pretreated by chemical precipitation of struvite yielded higher values of water flux, as the concentration of salt in the receiving solution increased and there was practically no infiltration of organic substances into the draw solution.
- The removal of the macromolecular fraction of organic compounds from the digestate took place mainly simultaneously with the chemical precipitation of struvite.
- An increase in the salt concentration of the draw solution, which allowed for a greater water recovery, resulted in the aggregation of the concentrated organic molecules in the feed solution.
- The correlation of the ζ potential with pH did not change when comparing raw digestate and concentrated samples (after process performance); pHIEP was about 1.9.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fuchs, W.; Drosg, B. Assessment of the State of the Art of Technologies for the Processing of Digestate Residue from Anaerobic Digesters. Water Sci. Technol. 2013, 67, 1984–1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabsch-Korbutowicz, M. Zastosowanie procesu wymuszonej osmozy do odsalania i odnowy wody. Ochr. Srodowiska 2016, 38, 9–14. [Google Scholar]
- Kucera, J. Desalination; Kucera, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; Volume 9781118208526, ISBN 9781118904855. [Google Scholar]
- ForwardOsmosisTech Guide to Forward Osmosis Membranes. Available online: www.forwardosmosistech.com/forward-osmosis-membranes (accessed on 17 July 2021).
- Li, L.; Shi, W.; Yu, S. Research on Forward Osmosis Membrane Technology Still Needs Improvement in Water Recovery and Wastewater Treatment. Water 2019, 12, 107. [Google Scholar] [CrossRef] [Green Version]
- Korenak, J.; Hélix-Nielsen, C.; Bukšek, H.; Petrinić, I. Efficiency and Economic Feasibility of Forward Osmosis in Textile Wastewater Treatment. J. Clean. Prod. 2019, 210, 1483–1495. [Google Scholar] [CrossRef]
- Jafarinejad, S. Forward Osmosis Membrane Technology for Nutrient Removal/Recovery from Wastewater: Recent Advances, Proposed Designs, and Future Directions. Chemosphere 2021, 263, 128116. [Google Scholar] [CrossRef]
- Corzo, B.; de la Torre, T.; Sans, C.; Escorihuela, R.; Navea, S.; Malfeito, J.J. Long-Term Evaluation of a Forward Osmosis-Nanofiltration Demonstration Plant for Wastewater Reuse in Agriculture. Chem. Eng. J. 2018, 338, 383–391. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W.; Johnson, D.; Hilal, N. Unlocking the Application Potential of Forward Osmosis through Integrated/Hybrid Process. Sci. Total Environ. 2020, 706, 136047. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Zou, S.; Zhang, B.; Wang, L.; He, Z. Forward Osmosis Promoted In-Situ Formation of Struvite with Simultaneous Water Recovery from Digested Swine Wastewater. Chem. Eng. J. 2018, 342, 274–280. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Xie, M.; Zhang, B.; Li, G.; Luo, W. Resource Recovery from Digested Manure Centrate: Comparison between Conventional and Aquaporin Thin-Film Composite Forward Osmosis Membranes. J. Membr. Sci. 2020, 593, 117436. [Google Scholar] [CrossRef]
- Ezugbe, E.; Kweinor Tetteh, E.; Rathilal, S.; Asante-Sackey, D.; Amo-Duodu, G. Desalination of Municipal Wastewater Using Forward Osmosis. Membranes 2021, 11, 119. [Google Scholar] [CrossRef]
- Jashrapuria, K.; Singh, S.P. Forward Osmosis in Desalination and Wastewater Treatment. In Pollution Control Technologies. Energy, Environment, and Sustainability; Singh, S.P., Rathinam, K., Gupta, T., Agarwal, A.K., Eds.; Springer: Singapore, 2021; pp. 157–175. [Google Scholar]
- Minier-Matar, J.; Hussain, A.; Janson, A.; Wang, R.; Fane, A.G.; Adham, S. Application of Forward Osmosis for Reducing Volume of Produced/Process Water from Oil and Gas Operations. Desalination 2015, 376, 1–8. [Google Scholar] [CrossRef]
- Ang, W.L.; Wahab Mohammad, A.; Johnson, D.; Hilal, N. Forward Osmosis Research Trends in Desalination and Wastewater Treatment: A Review of Research Trends over the Past Decade. J. Water Process. Eng. 2019, 31, 100886. [Google Scholar] [CrossRef]
- Camilleri-Rumbau, M.S.; Soler-Cabezas, J.L.; Christensen, K.V.; Norddahl, B.; Mendoza-Roca, J.A.; Vincent-Vela, M.C. Application of Aquaporin-Based Forward Osmosis Membranes for Processing of Digestate Liquid Fractions. Chem. Eng. J. 2019, 371, 583–592. [Google Scholar] [CrossRef]
- Shi, L.; Simplicio, W.S.; Wu, G.; Hu, Z.; Hu, H.; Zhan, X. Nutrient Recovery from Digestate of Anaerobic Digestion of Livestock Manure: A Review. Curr. Pollut. Rep. 2018, 4, 74–83. [Google Scholar] [CrossRef]
- Awad, A.M.; Jalab, R.; Minier-Matar, J.; Adham, S.; Nasser, M.S.; Judd, S.J. The Status of Forward Osmosis Technology Implementation. Desalination 2019, 461, 10–21. [Google Scholar] [CrossRef]
- Chekli, L.; Kim, Y.; Phuntsho, S.; Li, S.; Ghaffour, N.; Leiknes, T.; Shon, H.K. Evaluation of Fertilizer-Drawn Forward Osmosis for Sustainable Agriculture and Water Reuse in Arid Regions. J. Environ. Manag. 2017, 187, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Abdul Wahid, R.; Ang, W.L.; Mohammad, A.W.; Johnson, D.J.; Hilal, N. Evaluating Fertilizer-Drawn Forward Osmosis Performance in Treating Anaerobic Palm Oil Mill Effluent. Membranes 2021, 11, 566. [Google Scholar] [CrossRef]
- Nasr, P.; Sewilam, H. The Potential of Groundwater Desalination Using Forward Osmosis for Irrigation in Egypt. Clean Technol. Environ. Policy 2015, 17, 1883–1895. [Google Scholar] [CrossRef]
- Cath, T.Y.; Hancock, N.T.; Lundin, C.D.; Hoppe-Jones, C.; Drewes, J.E. A Multi-Barrier Osmotic Dilution Process for Simultaneous Desalination and Purification of Impaired Water. J. Membr. Sci. 2010, 362, 417–426. [Google Scholar] [CrossRef]
- Durdević, D.; Hulenić, I. Anaerobic Digestate Treatment Selection Model for Biogas Plant Costs and Emissions Reduction. Processes 2020, 8, 142. [Google Scholar] [CrossRef] [Green Version]
- Monfet, E.; Aubry, G.; Ramirez, A.A. Nutrient Removal and Recovery from Digestate: A Review of the Technology. Biofuels 2018, 9, 247–262. [Google Scholar] [CrossRef]
- Szymańska, M.; Szara, E.; Sosulski, T.; Wąs, A.; Van Pruissen, G.W.P.; Cornelissen, R.L.; Borowik, M.; Konkol, M. A Bio-Refinery Concept for n and p Recovery—A Chance for Biogas Plant Development. Energies 2019, 12, 155. [Google Scholar] [CrossRef] [Green Version]
- Baird, R.; Eaton, A.D.; Rice, E.W.; Bridgewater, L.; Federation, W.E. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017; ISBN 9780875532875. [Google Scholar]
- Chempur Karta Charakterystyki Substancji Chemicznej—Sodu Diwodorofosforan Bezwodny. Available online: http://chempur.pl/pliki/karty_charakterystyk/sodu_fosforan_I_bezwodny.pdf (accessed on 16 August 2021).
- Chempur Karta Charakterystyki Substancji Chemicznej—Chlorek Magnezu. Available online: http://chempur.pl/pliki/karty_charakterystyk/magnezu_chlorek_6h.pdf (accessed on 16 August 2021).
- Sterlitech Forward Osmosis (FO) Membranes. Available online: https://www.sterlitech.com/forward-osmosis-membranes.html (accessed on 22 June 2022).
- Urbanowska, A.; Polowczyk, I.; Kabsch-Korbutowicz, M. Struvite Precipitation from the Liquid Fraction of the Digestate of a Municipal Waste Biogas Plant. Environ. Prot. Eng. 2021, 47. [Google Scholar] [CrossRef]
- Cerrillo, M.; Palatsi, J.; Comas, J.; Vicens, J.; Bonmatí, A. Struvite Precipitation as a Technology to Be Integrated in a Manure Anaerobic Digestion Treatment Plant—Removal Efficiency, Crystal Characterization and Agricultural Assessment. J. Chem. Technol. Biotechnol. 2015, 90, 1135–1143. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Liu, X.-Y.; Zhang, H.-Q.; Wang, Y.; Xu, Y.-F.; Peng, B.-L.; Liu, Y. Modeling of Kinetic Characteristics of Alkaline-Surfactant-Polymer-Strengthened Foams Decay under Ultrasonic Standing Wave. Pet. Sci. 2022, 19, 1825–1839. [Google Scholar] [CrossRef]
- Holloway, R.; Childress, A.; Dennett, K.; Cath, T. Forward Osmosis for Concentration of Anaerobic Digester Centrate. Water Res. 2007, 41, 4005–4014. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, T.; Chu, H.; Zhang, W.; Huang, W.; Dong, B.; Wu, D.; Chen, F. Natural Organic Matter Separation by Forward Osmosis: Performance and Mechanisms. Water Res. 2021, 191, 116829. [Google Scholar] [CrossRef]
- Almoalimi, K.; Liu, Y.-Q. Fouling and Cleaning of Thin Film Composite Forward Osmosis Membrane Treating Municipal Wastewater for Resource Recovery. Chemosphere 2022, 288, 132507. [Google Scholar] [CrossRef]
- Xie, M.; Nghiem, L.D.; Price, W.E.; Elimelech, M. Comparison of the Removal of Hydrophobic Trace Organic Contaminants by Forward Osmosis and Reverse Osmosis. Water Res. 2012, 46, 2683–2692. [Google Scholar] [CrossRef] [Green Version]
- Israelachvili, J.N. Intermolecular and Surface Forces; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780123919274. [Google Scholar]
- Liu, X.; Wu, J.; Liu, C.; Wang, J. Removal of Cobalt Ions from Aqueous Solution by Forward Osmosis. Sep. Purif. Technol. 2017, 177, 8–20. [Google Scholar] [CrossRef]
- Eddouibi, J.; Abderafi, S.; Vaudreuil, S.; Bounahmidi, T. Water Desalination by Forward Osmosis: Dynamic Performance Assessment and Experimental Validation Using MgCl2 and NaCl as Draw Solutes. Comput. Chem. Eng. 2021, 149, 107313. [Google Scholar] [CrossRef]
- Phillip, W.A.; Yong, J.S.; Elimelech, M. Reverse Draw Solute Permeation in Forward Osmosis: Modeling and Experiments. Environ. Sci. Technol. 2010, 44, 5170–5176. [Google Scholar] [CrossRef]
- Hickenbottom, K.L.; Hancock, N.T.; Hutchings, N.R.; Appleton, E.W.; Beaudry, E.G.; Xu, P.; Cath, T.Y. Forward Osmosis Treatment of Drilling Mud and Fracturing Wastewater from Oil and Gas Operations. Desalination 2013, 312, 60–66. [Google Scholar] [CrossRef]
- Jurgutis, L.; Šlepetienė, A.; Šlepetys, J.; Cesevičienė, J. Towards a Full Circular Economy in Biogas Plants: Sustainable Management of Digestate for Growing Biomass Feedstocks and Use as Biofertilizer. Energies 2021, 14, 4272. [Google Scholar] [CrossRef]
- Peng, W.; Pivato, A. Sustainable Management of Digestate from the Organic Fraction of Municipal Solid Waste and Food Waste Under the Concepts of Back to Earth Alternatives and Circular Economy. Waste Biomass Valorization 2019, 10, 465–481. [Google Scholar] [CrossRef]
- Chiumenti, A.; da Borso, F.; Chiumenti, R.; Teri, F.; Segantin, P. Treatment of Digestate from a Co-Digestion Biogas Plant by Means of Vacuum Evaporation: Tests for Process Optimization and Environmental Sustainability. Waste Manag. 2013, 33, 1339–1344. [Google Scholar] [CrossRef]
- Gienau, T.; Brüß, U.; Kraume, M.; Rosenberger, S. Nutrient Recovery from Biogas Digestate by Optimised Membrane Treatment. Waste Biomass Valorization 2018, 9, 2337–2347. [Google Scholar] [CrossRef]
- Ramprasad, C.; Alekhya, D.; Bhishmitha, C.; Deepika, C.S. Precipitation of Struvite by Sustainable Waste Materials and Use as Slow Release Fertilizer—A Circular Economy Approach. IOP Conf. Ser. Mater. Sci. Eng. 2020, 955, 012088. [Google Scholar] [CrossRef]
- Achilleos, P.; Roberts, K.R.; Williams, I.D. Struvite Precipitation within Wastewater Treatment: A Problem or a Circular Economy Opportunity? Heliyon 2022, 8, e09862. [Google Scholar] [CrossRef]












| Index | Value |
|---|---|
| pH | 7.23 |
| Temperature, °C | 21 |
| Conductivity, mS/cm | 20.3 |
| Dry residue, mg/dm3 | 55,820 |
| Alkalinity, mmol/dm3 | 150 |
| Total hardness, mval/dm3 | 753 |
| Chemical oxygen demand (COD), mg O2/dm3 | 11,450 |
| 5-days biochemical oxygen demand (BOD5), mg O2/dm3 | 3600 |
| Dissolved organic carbon (DOC), mg C/dm3 | 4210 |
| N-NH4+, mg/dm3 | 776 |
| N-NO2−, mg/dm3 | 5.9 |
| N-NO3−, mg/dm3 | below level of detection |
| PO43−, mg/dm3 | 21.2 |
| Mg, mg/dm3 | 235 |
| Ca, mg/dm3 | 420 |
| K, mg/dm3 | 3220 |
| Li, mg/dm3 | 7 |
| P, mg/dm3 | 21.4 |
| Magnesium Chloride | Monosodium Phosphate | |
|---|---|---|
| manufacturer | Chempur | |
| chemical formula | MgCl2 | NaH2PO4 |
| molar mass, g/mol | 95.211 | 119.98 |
| form | solid | solid |
| color | colorless to white | white to colorless |
| odour | odourless | n.a. |
| pH | 5–6.5 (5%, 20 °C) | 4–4.5 (5%, 20 °C) |
| density, g/cm3 | 1.57 (20 °C) | 1.91 (20 °C) |
| solubility in water, g/dm3 | 2430 (20 °C) | n.a. |
| Index | Value |
|---|---|
| Conductivity, mS/cm | 19.7 |
| pH | 9.0 |
| COD, mg O2/dm3 | 10,360 |
| BOD5, mg O2/dm3 | 3510 |
| DOC, mg C/dm3 | 3990 |
| N-NH4+, mg/dm3 | 772 |
| Mg, mg/dm3 | 23.1 |
| Ca, mg/dm3 | 42.3 |
| K, mg/dm3 | 2970 |
| Li, mg/dm3 | 6.9 |
| P, mg/dm3 | 1.9 |
| NaCl Concentration | Osmotic Pressure | |
|---|---|---|
| [mol/dm3] | [MPa] | [atm] |
| 0.5 | 2.478 | 24.46 |
| 1 | 4.985 | 48.93 |
| 3 | 14.87 | 146.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbanowska, A.; Polowczyk, I.; Kabsch-Korbutowicz, M. Treatment of Liquid Fraction of Digestate by Integrated Process Struvite Precipitation—Forward Osmosis. Energies 2023, 16, 47. https://doi.org/10.3390/en16010047
Urbanowska A, Polowczyk I, Kabsch-Korbutowicz M. Treatment of Liquid Fraction of Digestate by Integrated Process Struvite Precipitation—Forward Osmosis. Energies. 2023; 16(1):47. https://doi.org/10.3390/en16010047
Chicago/Turabian StyleUrbanowska, Agnieszka, Izabela Polowczyk, and Małgorzata Kabsch-Korbutowicz. 2023. "Treatment of Liquid Fraction of Digestate by Integrated Process Struvite Precipitation—Forward Osmosis" Energies 16, no. 1: 47. https://doi.org/10.3390/en16010047
APA StyleUrbanowska, A., Polowczyk, I., & Kabsch-Korbutowicz, M. (2023). Treatment of Liquid Fraction of Digestate by Integrated Process Struvite Precipitation—Forward Osmosis. Energies, 16(1), 47. https://doi.org/10.3390/en16010047