Supercritical Transesterification of Palm Oil and Hydrated Ethanol in a Fixed Bed Reactor with a CaO/Al2O3 Catalyst
Abstract
:1. Introduction
2. Experimental Section
2.1. Catalyst Preparation
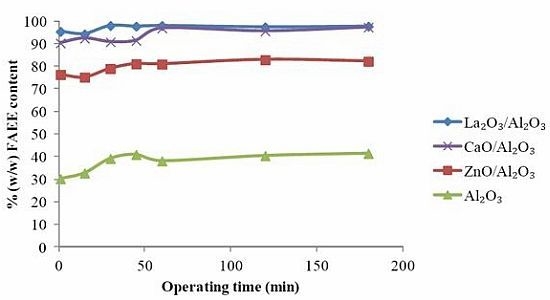
2.2. Catalyst Characterization
2.3. Transesterification Apparatus and Procedure

2.4. Biodiesel Sample Analysis
2.5. Experimental Design for Optimization Study
3. Results and Discussion
3.1. The Preliminary Study
3.1.1. Effects of Temperature on the FAEE Content


3.1.2. Effect of Pressure on the % (w/w) FAEE Content
| Peak # | Retention time (min) | Name |
|---|---|---|
| 1 | 2.60 | Butanoic acid |
| 2 | 5.36 | 5-Hexenoic acid |
| 3 | 5.57 | Ethyl hexanoate |
| 4 | 6.80 | 3-Heptenoic acid |
| 5 | 6.92 | Heptanoic acid |
| 6 | 7.00 | Ethyl 6-heptenoate |
| 7 | 7.06 | 1-Undecene |
| 8 | 7.15 | Ethyl heptanoate |
| 9 | 7.28 | 5-Undecene |
| 10 | 8.53 | Ethyl 7-octenoate |
| 11 | 8.59 | 1-Tridecene |
| 12 | 8.67 | Ethyl octanoate |
| 13 | 9.97 | Ethyl 8-nonenoate |
| 14 | 10.04 | 1-Tridecene |
| 15 | 10.09 | Ethyl nonanoate |
| 16 | 10.15 | Pentadecane |
| 17 | 11.31 | Ethyl 9-decenoate |
| 18 | 11.38 | 1-Pentadecene |
| 19 | 12.19 | Cyclopentene |
| 20 | 12.34 | 3-Hexyl-1-cyclohexene |
| 21 | 12.58 | Ethyl undecenoate |
| 22 | 12.65 | 1-Pentadecene |
| 23 | 12.75 | Pentadecane |
| 24 | 13.53 | Dodecanoic acid |
| 25 | 13.68 | 5-Tetradecen-1-ol |
| 26 | 13.97 | Ethyl dodecanoate |
| 27 | 15.49 | Ethyl 9-hexadecenoate |
| 28 | 17.12 | Tetradecanoic acid |
| 29 | 17.30 | 9-Octadecen-1-ol |
| 30 | 17.99 | Ethyl tetradecanoate |
| 31 | 22.92 | Methyl hexadecanoate |
| 32 | 25.52 | Hexanoic acid |
| 33 | 27.09 | Ethyl hexadecanoate |
| 34 | 35.14 | Methyl 9-octadecenoate |
| 35 | 40.80 | cis-9-Octadecenoic acid |
| 36 | 41.40 | cis-9-Hexadecenal |
| 37 | 42.12 | Ethyl 9,12-octadecadienoate |
| 38 | 43.58 | Ethyl 9-octadecenoate |
| 39 | 44.54 | 3,13-Octadecedien-1-ol |
| 40 | 47.85 | Ethyloctadecanoate |

3.1.3. Effects of the Ethanol/Palm Oil Molar Ratio on the % (w/w) FAEE Content
3.1.4. Effect of the Residence Time on the % (w/w) FAEE Content


3.2. The Optimization Study
| Run order | Temperature (°C) | Pressure (MPa) | Total mass flow rate (g/min) | EtOH:Oil (mol ratio) | FAEE% (w/w) |
|---|---|---|---|---|---|
| 3 | 238 | 8.12 | 2.01 | 18 | 42.8 |
| 26 | 243 | 8.42 | 1.99 | 18 | 46.4 |
| 24 | 284 | 8.18 | 2.00 | 18 | 59.4 |
| 37 | 280 | 7.88 | 2.05 | 18 | 53.7 |
| 41 | 241 | 20.00 | 2.02 | 18 | 56.1 |
| 14 | 242 | 19.82 | 2.01 | 18 | 54.0 |
| 11 | 285 | 20.72 | 1.99 | 18 | 66.3 |
| 29 | 284 | 20.42 | 2.02 | 18 | 69.0 |
| 16 | 262 | 14.00 | 3.06 | 18 | 15.8 |
| 36 | 238 | 7.82 | 4.03 | 18 | 7.5 |
| 21 | 240 | 8.12 | 4.01 | 18 | 7.0 |
| 10 | 283 | 8.42 | 3.98 | 18 | 48.4 |
| 34 | 278 | 8.12 | 4.02 | 18 | 49.0 |
| 31 | 237 | 19.58 | 4.03 | 18 | 19.8 |
| 30 | 241 | 20.12 | 4.01 | 18 | 23.7 |
| 42 | 284 | 20.30 | 4.03 | 18 | 60.0 |
| 28 | 281 | 20.12 | 4.00 | 18 | 54.2 |
| 13 | 259 | 14.30 | 2.01 | 24 | 36.0 |
| 17 | 243 | 14.48 | 3.03 | 24 | 28.2 |
| 43 | 281 | 14.18 | 2.99 | 24 | 57.2 |
| 12 | 257 | 8.30 | 3.01 | 24 | 20.7 |
| 2 | 261 | 19.88 | 3.03 | 24 | 42.7 |
| 8 | 263 | 13.82 | 3.03 | 24 | 27.5 |
| 1 | 259 | 14.30 | 3.00 | 24 | 25.2 |
| 5 | 262 | 13.70 | 2.98 | 24 | 26.5 |
| 27 | 260 | 20.36 | 4.03 | 24 | 19.1 |
| 7 | 244 | 8.30 | 2.06 | 30 | 51.0 |
| 4 | 240 | 8.18 | 2.01 | 30 | 47.3 |
| 15 | 280 | 8.00 | 2.04 | 30 | 80.6 |
| 32 | 284 | 8.42 | 2.06 | 30 | 85.9 |
| 9 | 248 | 20.42 | 2.03 | 30 | 73.0 |
| 18 | 242 | 19.82 | 2.00 | 30 | 70.0 |
| 19 | 284 | 20.78 | 2.01 | 30 | 97.4 |
| 39 | 278 | 19.88 | 2.04 | 30 | 90.0 |
| 35 | 260 | 14.18 | 3.04 | 30 | 31.8 |
| 38 | 238 | 8.48 | 4.05 | 30 | 35.0 |
| 20 | 243 | 8.12 | 4.02 | 30 | 40.7 |
| 33 | 278 | 8.30 | 4.03 | 30 | 59.4 |
| 23 | 283 | 8.12 | 4.01 | 30 | 64.6 |
| 6 | 237 | 19.82 | 4.06 | 30 | 41.2 |
| 40 | 241 | 20.00 | 4.03 | 30 | 45.1 |
| 22 | 279 | 20.00 | 4.01 | 30 | 50.8 |
| 25 | 283 | 20.60 | 3.99 | 30 | 55.0 |
| Source | Sum of squares | DF | Mean square | F value | P-value |
|---|---|---|---|---|---|
| A | 3041.85 | 1 | 3041.85 | 60.62 | <0.0001 |
| B | 618.93 | 1 | 618.93 | 12.33 | 0.0015 |
| C | 4541.78 | 1 | 4541.78 | 90.52 | <0.0001 |
| D | 2807.72 | 1 | 2807.72 | 55.96 | <0.0001 |
| A2 | 503.35 | 1 | 503.35 | 10.03 | 0.0037 |
| B2 | 185.73 | 1 | 185.73 | 3.70 | 0.0446 |
| C2 | 72.56 | 1 | 72.56 | 1.44 | 0.2392 * |
| D2 | 2.09 | 1 | 2.09 | 0.04 | 0.8398 * |
| AB | 316.09 | 1 | 316.09 | 6.29 | 0.0181 |
| AC | 285.95 | 1 | 285.95 | 5.69 | 0.0240 |
| AD | 5.49 | 1 | 5.49 | 0.10 | 0.7432 * |
| BC | 187.05 | 1 | 187.05 | 3.72 | 0.0637 * |
| BD | 10.42 | 1 | 10.42 | 0.21 | 0.6520 * |
| CD | 46.89 | 1 | 46.89 | 0.93 | 0.3420 * |
| Residual | 1404.92 | 28 | 50.18 | ||
| Core Total | 19442.97 | 42 |


3.3. Fuel Properties Analysis
| Physical-Chemical Properties | Samples | Biodiesel Standard Specification | |
|---|---|---|---|
| FAME | FAEE | ||
| % (w/w) ester content | 98.2 | 97.0 | 96.5 |
| Density at 20 °C (kg/m3) | 865 | 883 | 860–900 |
| Viscosity at 40 °C (mm2/s) | 4.1 | 4.8 | 3.5–5.0 |
| Flash point (°C) | 110.0 | 117.0 | 120 (min) |
| Cetane index | 56 | 57 | 51 (min) |
| Distillation characteristics (°C) | |||
| IBP | 187.3 | 190.2 | Take note |
| 50% | 308.2 | 310.2 | 245.0–310.0 |
| 95% | 310.6 | 313.4 | 370.0 (max) |
| FBP | 501.1 | 503.2 | Take note |
| Pour point (°C) | 11 | 6 | Take note |
| Acid value (mg KOH) | 0.12 | 0.14 | 0.50 |
| Copper corrosion | 1A | 1A | Class 1A (max) |
| Free glycerin content (%) | N/D | N/D | 0.02 (max) |
| Total glycerin content (%) | 0.11 | 0.18 | 0.25 (max) |
4. Conclusions
Acknowledgments
References
- Pinnarat, T.; Savage, P.E. Assessment of noncatalytic biodiesel synthesis using supercritical reaction conditions. Ind. Eng. Chem. Res. 2008, 47, 6801–6808. [Google Scholar] [CrossRef]
- Sawangkeaw, R.; Bunyakiat, K.; Ngamprasertsith, S. A review of laboratory-scale research on lipid conversion to biodiesel with supercritical methanol (2001–2009). J. Supercrit. Fluids 2010, 55, 1–13. [Google Scholar] [CrossRef]
- Lee, J.S.; Saka, S. Biodiesel production by heterogeneous catalysts and supercritical technologies. Bioresour. Technol. 2010, 101, 7191–7200. [Google Scholar] [CrossRef] [PubMed]
- Juan, J.C.; Kartika, D.A.; Wu, T.Y.; Hin, T.Y.Y. Biodiesel production from Jatropha oil by catalytic and non-catalytic approaches: An overview. Bioresour. Technol. 2010, 102, 452–460. [Google Scholar] [CrossRef] [PubMed]
- De Boer, K.; Bahri, P.A. Supercritical methanol for fatty acid methyl ester production: A review. Biomass Bioenergy 2011, 35, 983–991. [Google Scholar]
- Trentin, C.M.; Lima, A.P.; Alkimim, I.P.; da Silva, C.; de Castilhos, F.; Mazutti, M.A.; Oliveira, J.V. Continuous catalyst-free production of fatty acid ethyl esters from soybean oil in microtube reactor using supercritical carbon dioxide as co-solvent. J. Supercrit. Fluids 2011, 56, 283–291. [Google Scholar] [CrossRef]
- Vieitez, I.; Pardo, M.J.; da Silva, C.; Bertoldi, C.; de Castilhos, F.; Oliveira, J.V.; Grompone, M.A.; Jachmanián, I. Continuous synthesis of castor oil ethyl esters under supercritical ethanol. J. Supercrit. Fluids 2011, 56, 271–276. [Google Scholar] [CrossRef]
- Saka, S.; Kusdiana, D. Biodiesel fuel from rapeseed oil as prepared in supercritical methanol. Fuel 2001, 80, 225–231. [Google Scholar] [CrossRef]
- Kusdiana, D.; Saka, S. Kinetics of transesterification in rapeseed oil to biodiesel fuel as treated in supercritical methanol. Fuel 2001, 80, 693–698. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel from vegetable oils via transesterification in supercritical methanol. Energy Convers. Manag. 2002, 43, 2349–2356. [Google Scholar] [CrossRef]
- Boey, P.L.; Maniam, G.P.; Hamid, S.A. Performance of calcium oxide as a heterogeneous catalyst in biodiesel production: A review. Chem. Eng. J. 2011, 168, 15–22. [Google Scholar] [CrossRef]
- Zabeti, M.; Daud, W.M.A.W.; Aroua, M.K. Biodiesel production using alumina-supported calcium oxide: An optimization study. Fuel Process. Technol. 2010, 91, 243–248. [Google Scholar] [CrossRef]
- Kouzu, M.; Kasuno, T.; Tajika, M.; Sugimoto, Y.; Yamanaka, S.; Hidaka, J. Calcium oxide as a solid base catalyst for transesterification of soybean oil and its application to biodiesel production. Fuel 2008, 87, 2798–2806. [Google Scholar] [CrossRef]
- Ngamcharussrivichai, C.; Nunthasanti, P.; Tanachai, S.; Bunyakiat, K. Biodiesel production through transesterification over natural calciums. Fuel Process. Technol. 2010, 91, 1409–1415. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel from sunflower oil in supercritical methanol with calcium oxide. Energy Convers. Manag. 2007, 48, 937–941. [Google Scholar] [CrossRef]
- Montgomery, C.D. Design and Analysis of Experiments, 7th ed.; John Wiley and Sons: New York, NY, USA, 2010; p. 428. [Google Scholar]
- Box, G.E.P.; Hunter, J.S.; Hunter, W.G. Statistics for Experimenters: Design, Innovation and Discovery, 2nd ed.; Wiley-Interscience: New Jersey, NJ, USA, 2005; pp. 450–451. [Google Scholar]
- Abdurashidova, A.; Bazaev, A.; Bazaev, E.; Abdulagatov, I. The thermal properties of water-ethanol system in the near-critical and supercritical states. High Temp. 2007, 45, 178–186. [Google Scholar] [CrossRef]
- Anand, K.; Ranjan, A.; Mehta, P.S. Predicting the density of straight and processed vegetable Oils from fatty acid composition. Energy Fuels 2010, 24, 3262–3266. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, J.; Chen, J.; Sun, Y. Biofuel production from catalytic cracking of woody oils. Bioresour. Technol. 2010, 101, 5586–5591. [Google Scholar] [CrossRef] [PubMed]
- Megahed, O.; Abdelmonem, N.; Nabil, D. Thermal cracking of rapeseed oil as alternative fuel. Energy Sources Part A 2004, 26, 1033–1042. [Google Scholar] [CrossRef]
- Vieitez, I.; da Silva, C.; Borges, G.R.; Corazza, F.C.; Oliveira, J.V.; Grompone, M.A.; Jachmanian, I. Continuous production of soybean biodiesel in supercritical ethanol/water mixtures. Energy Fuels 2008, 22, 2805–2809. [Google Scholar] [CrossRef]
- Bunyakiat, K.; Makmee, S.; Sawangkeaw, R.; Ngamprasertsith, S. Continuous production of biodiesel via transesterification from vegetable oils in supercritical methanol. Energy Fuels 2006, 20, 812–817. [Google Scholar] [CrossRef]
- Song, E.S.; Lim, J.W.; Lee, H.S.; Lee, Y.W. Transesterification of RBD palm oil using supercritical methanol. J. Supercrit. Fluids 2008, 44, 356–363. [Google Scholar] [CrossRef]
- Gui, M.M.; Lee, K.T.; Bhatia, S. Supercritical ethanol technology for the production of biodiesel: Process optimization studies. J. Supercrit. Fluids 2009, 49, 286–292. [Google Scholar] [CrossRef]
- Hoffmann, M.M.; Conradi, M.S. Are there hydrogen bonds in supercritical methanol and ethanol? J. Phys. Chem. B 1998, 102, 263–271. [Google Scholar] [CrossRef]
- Velez, A.; Hegel, P.; Mabe, G.; Brignole, E.A. Density and conversion in biodiesel production with supercritical methanol. Ind. Eng. Chem. Res. 2010, 49, 7666–7670. [Google Scholar] [CrossRef]
- Bertoldi, C.; da Silva, C.; Bernardon, J.P.; Corazza, M.L.; Filho, L.C.; Oliveira, J.V.; Corazza, F.C. Continuous production of biodiesel from soybean oil in supercritical ethanol and carbon dioxide as cosolvent. Energy Fuels 2009, 23, 5165–5172. [Google Scholar] [CrossRef]
- Chemicalland21 Homepage. Available online: http://chemicalland21.com (accessed on 6 January 2012).
- Knothe, G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process. Technol. 2005, 86, 1059–1070. [Google Scholar] [CrossRef]
- Marchetti, J.M.; Errazu, A.F. Technoeconomic study of supercritical biodiesel production plant. Energy Convers. Manag. 2008, 49, 2160–2164. [Google Scholar] [CrossRef]
- Diaz, M.S.; Espinosa, S.; Brignole, E.A. Model-Based cost minimization in noncatalytic biodiesel production plants. Energy Fuels 2009, 23, 5587–5595. [Google Scholar] [CrossRef]
- Deshpande, A.; Anitescu, G.; Rice, P.A.; Tavlarides, L.L. Supercritical biodiesel production and power cogeneration: Technical and economic feasibilities. Bioresour. Technol. 2010, 101, 1834–1843. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sawangkeaw, R.; Tejvirat, P.; Ngamcharassrivichai, C.; Ngamprasertsith, S. Supercritical Transesterification of Palm Oil and Hydrated Ethanol in a Fixed Bed Reactor with a CaO/Al2O3 Catalyst. Energies 2012, 5, 1062-1080. https://doi.org/10.3390/en5041062
Sawangkeaw R, Tejvirat P, Ngamcharassrivichai C, Ngamprasertsith S. Supercritical Transesterification of Palm Oil and Hydrated Ethanol in a Fixed Bed Reactor with a CaO/Al2O3 Catalyst. Energies. 2012; 5(4):1062-1080. https://doi.org/10.3390/en5041062
Chicago/Turabian StyleSawangkeaw, Ruengwit, Pornicha Tejvirat, Chawalit Ngamcharassrivichai, and Somkiat Ngamprasertsith. 2012. "Supercritical Transesterification of Palm Oil and Hydrated Ethanol in a Fixed Bed Reactor with a CaO/Al2O3 Catalyst" Energies 5, no. 4: 1062-1080. https://doi.org/10.3390/en5041062
APA StyleSawangkeaw, R., Tejvirat, P., Ngamcharassrivichai, C., & Ngamprasertsith, S. (2012). Supercritical Transesterification of Palm Oil and Hydrated Ethanol in a Fixed Bed Reactor with a CaO/Al2O3 Catalyst. Energies, 5(4), 1062-1080. https://doi.org/10.3390/en5041062