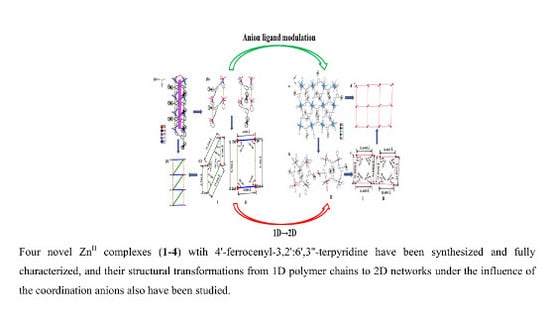
Four Novel Zn (II) Coordination Polymers Based on 4′-Ferrocenyl-3,2′:6′,3′′-Terpyridine: Engineering a Switch from 1D Helical Polymer Chain to 2D Network by Coordination Anion Modulation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Methods
2.2. X-ray Crystallography
2.3. Synthesis and Characterization of Compounds
3. Results and Discussion
3.1. Syntheses
3.2. Description of Crystal Structures
3.2.1. Structure of the Ligand
3.2.2. Structure of Complexes 1–4
3.3. UV-Vis Absorption and Optical Band Gap
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Housecroft, C.E. 4,2′:6′,4″-Terpyridines: Diverging and diverse building blocks in coordination polymers and metallomacrocycles. Dalton Trans. 2014, 43, 6594–6604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Housecroft, C.E. Divergent 4,2′:6′,4′- and 3,2′:6′,3″-terpyridines as linkers in 2- and 3-dimensional architectures. CrystEngComm 2015, 17, 7461–7468. [Google Scholar] [CrossRef] [Green Version]
- Schubert, U.S.; Hofmeier, H.; Newkome, G.R. Modern Terpyridine Chemistry, 1st ed.; WILEY-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2006; ISBN 978-3-527-31475-1. [Google Scholar]
- Klein, Y.M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. 4,2′:6′,4″- and 3,2′:6′,3″-Terpyridines: The Conflict between Well-Defined Vectorial Properties and Serendipity in the Assembly of 1D-, 2D- and 3D-Architectures. Materials 2017, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Khatua, S.; Goswami, S.; Biswas, S.; Tomar, K.; Jena, H.S.; Konar, S. Stable Multiresponsive Luminescent MOF for Colorimetric Detection of Small Molecules in Selective and Reversible Manner. Chem. Mater. 2015, 27, 5349–5360. [Google Scholar] [CrossRef]
- Chen, N.; Li, M.X.; Yang, P.; He, X.; Shao, M.; Zhu, S.R. Chiral Coordination Polymers with SHG-Active and Luminescence: An Unusual Homochiral 3D MOF Constructed from Achiral Components. Cryst. Growth Des. 2013, 13, 2650–2660. [Google Scholar] [CrossRef]
- Schubert, U.S.; Winter, A.; Newkome, G.R. Terpyridine-Based Materials, 1st ed.; Wiley-VCH Verlag & Co., KGaA: Weinheim, Germany, 2011; ISBN 978-3-527-33038-6. [Google Scholar]
- Constable, E.C. 2,2′:6′,2″-terpyridines: From chemical obscurity to common supramolecular motifs. Chem. Soc. Rev. 2007, 36, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Butler, I.R.; McDonald, S.J.; Hursthouse, M.B.; Abdul Malik, K.M. Ferrocenylpyridines: A new synthesis of 4′-ferrocenylterpyridine and the single crystal structure of a C3-ferrocenophane, [(η-C5H4CHCH2C(O)2-C5H4N)2CHC(O)2-C5H4N]Fe. Polyhedron 1995, 14, 529–539. [Google Scholar] [CrossRef]
- Barquín, M.; Cancela, J.; González Garmendia, M.J.; Quintanilla, J.; Amador, U. Coordination compounds of 4,2′-6′,4″-terpyridine, [MCl2(4,2′-6′,4″-terpyridine)], M = Mn (II), Co (II), Ni (II), Cu (II) or Zn (II). Crystal structure of catena-poly [(dichlorozinc)-μ-(4,2′-6′,4″-terpyridine)]. Polyhedron 1998, 17, 2373–2378. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Neuburger, M.; Vujovic, S.; Zampese, J.A.; Zhang, G. Cobalt (II) coordination polymers with 4′-substituted 4,2′:6′,4″- and 3,2′:6′,3″-terpyridines: Engineering a switch from planar to undulating chains and sheets. CrystEngComm 2012, 14, 3554–3563. [Google Scholar] [CrossRef] [Green Version]
- Nijs, T.; Malzner, F.J.; Fatayer, S.; Wackerlin, A.; Nowakowska, S.; Constable, E.C.; Housecroft, C.E.; Jung, T.A. Programmed assembly of 4,2′:6′,4″-terpyridine derivatives into porous, on-surface networks. Chem. Commun. 2015, 51, 12297–12300. [Google Scholar] [CrossRef] [PubMed]
- Maximilian Klein, Y.; Constable, E.C.; Housecroft, C.E.; Prescimone, A. A 3-dimensional {42·84}lvt net built from a ditopic bis(3,2′:6′,3″-terpyridine) tecton bearing long alkyl tails. CrystEngComm 2015, 17, 2070–2073. [Google Scholar] [CrossRef] [Green Version]
- Granifo, J.; Vargas, M.; Garland, M.T.; Ibáñez, A.; Gaviño, R.; Baggio, R. The novel ligand 4′-phenyl-3,2′:6′,3″-terpyridine (L) and the supramolecular structure of the dinuclear complex [Zn2(μ-L)(acac)4]·H2O (acac = acetylacetonato). Inorg. Chem. Commun. 2008, 11, 1388–1391. [Google Scholar] [CrossRef]
- Wang, B.C.; Chen, X.L.; Hu, H.M.; Yao, H.L.; Xue, G.L. Two novel Zn (II) coordination polymers based on trigonal ligand: 4′-(4-pyridyl)-3,2′:6′,3″-terpyridine. Inorg. Chem. Commun. 2009, 12, 856–859. [Google Scholar] [CrossRef]
- Yang, P.; Wang, M.S.; Shen, J.J.; Li, M.X.; Wang, Z.X.; Shao, M.; He, X. Seven novel coordination polymers constructed by rigid 4-(4-carboxyphenyl)-terpyridine ligands: Synthesis, structural diversity, luminescence and magnetic properties. Dalton Trans. 2014, 43, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Tan, J.; Su, J.; Zhang, J.; Zhang, S.; Wu, J.; Tian, Y. Syntheses, crystal structures and third-order nonlinear optical properties of two series of Zn (II) complexes using the thiophene-based terpyridine ligands. Dyes Pigments 2016, 130, 216–225. [Google Scholar] [CrossRef]
- Klein, Y.M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Coordination Behaviour of 1-(4,2′:6′,4″-terpyridin-4′-yl) ferrocene and 1-(3,2′:6′,3″-terpyridin-4′-yl) ferrocene: Predictable and Unpredictable Assembly Algorithms. Aust. J. Chem. 2016, 70, 468–477. [Google Scholar] [CrossRef]
- Zhang, L.; Li, C.J.; He, J.E.; Chen, Y.Y.; Zheng, S.R.; Fan, J.; Zhang, W.G. Construction of New Coordination Polymers from 4′-(2,4-disulfophenyl)-3,2′:6′,3″-terpyridine: Polymorphism, pH-dependent syntheses, structures, and properties. J. Solid State Chem. 2016, 233, 444–454. [Google Scholar] [CrossRef]
- Wang, X.C.; Tian, Y.P.; Kan, Y.H.; Zuo, C.Y.; Wu, J.Y.; Jin, B.K.; Zhou, H.P.; Yang, J.X.; Zhang, S.Y.; Tao, X.T.; et al. Functionalized ferrocenes and ferroceniums: Synthesis, crystal structures and electrochemical properties based on carbazole/phenothiazine-ferrocene conjugated molecules. Dalton Trans. 2009, 21, 4096–4103. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.F.; Zhu, L.Y.; Zeng, Q.L.; Liu, Q.S.; Zhang, J.; Li, S.L.; Zhou, H.P.; Zhang, S.Y.; Wu, J.Y.; Tian, Y.P. Novel metal-organic hybrid materials constructed by ferrocenyl terpyridine derivatives and ZnIIX2 (X = Cl−, Br−, I−, SCN− and CH3COO−). J. Organomet. Chem. 2015, 789–790, 22–28. [Google Scholar] [CrossRef]
- Farlow, B.; Nile, T.A.; Walsh, J.L.; McPhail, A.T. Synthesis, X-ray structural determination and coordination chemistry of 4′-Ferrocenyl-2,2′:6′,2″-terpyridine. Polyhedron 1993, 12, 2891–2894. [Google Scholar] [CrossRef]
- Constable, E.C.; Edwards, A.J.; Martinez-Manez, R.; Raithby, P.R.; Thompson, A.M.C. Complexes containing Ferrocenyl Groups as Redox Spectators; Synthesis, Molecular Structure and Coordination Behaviour of 4′-Ferrocenyl-2,2′:6′,2″-terpyridine. J. Chem. Soc. Dalton Trans. 1994, 645–650. [Google Scholar] [CrossRef]
- Liu, B.; Yang, J.; Yang, G.C.; Ma, J.F. Four new three-dimensional polyoxometalate-based metal-organic frameworks constructed from[Mo6O18(O3AsPh)24− polyoxoanions and copper(I)-organic fragments: Syntheses, structures, electrochemistry, and photocatalysis properties. Inorg. Chem. 2013, 52, 84–94. [Google Scholar] [CrossRef] [PubMed]









| Compounds | L | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|
| Empirical Formula | C25H19FeN3 | C50H38Cl4Fe2 N6O3Zn2 | C51H46Br4Fe2N6 O3Zn2 | C50H38Fe2I4N6Zn2 | C162H120Cl18Fe6 N24S6Zn3 |
| Formula Weight | 417.28 | 1155.10 | 1353.02 | 1472.90 | 3764.49 |
| Crystal System | Orthorhombic | Triclinic | Triclinic | Triclinic | Monoclinic |
| Space Group | P212121 | Pī | Pī | Pī | P21/n |
| a(Å) | 10.027(8) | 8.700(5) | 8.901(5) | 9.1633(18) | 15.271(5) |
| b(Å) | 11.335(9) | 16.897(5) | 17.336(5) | 16.802(3) | 16.110(5) |
| c(Å) | 16.980(14) | 17.913(5) | 18.223(5) | 18.237(4) | 32.652(5) |
| a[°] | 90.00 | 76.423(5) | 72.998(5) | 71.159(2) | 90.000(5) |
| b[°] | 90.00 | 83.211(5) | 80.299(5) | 77.289(2) | 100.019(5) |
| γ[°] | 90.00 | 85.013(5) | 82.664(5) | 81.326(2) | 90.000(5) |
| V(Å3) | 1930(3) | 2537.0(18) | 2641.4(18) | 2582.4(9) | 7910(4) |
| Z | 4 | 2 | 2 | 2 | 2 |
| R1, wR2 | 0.0372 | 0.0477 | 0.0589 | 0.0369 | 0.0679 |
| [I ≥ 2σ (I)] | 0.0999 | 0.1481 | 0.1684 | 0.1127 | 0.1778 |
| R1, wR2 | 0.0447 | 0.0657 | 0.0978 | 0.0512 | 0.0890 |
| [all data] | 0.1045 | 0.1640 | 0.1867 | 0.1225 | 0.1960 |
| S on F2 | 1.068 | 1.071 | 1.068 | 1.057 | 1.049 |
| CCDC | 1,012,997 | 1,407,410 | 1,045,348 | 1,439,156 | 1,407,959 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, L.; Wu, D.; Wang, X.; Du, W.; Zhang, J.; Li, S.; Zhou, H.; Wu, J.; Tian, Y. Four Novel Zn (II) Coordination Polymers Based on 4′-Ferrocenyl-3,2′:6′,3′′-Terpyridine: Engineering a Switch from 1D Helical Polymer Chain to 2D Network by Coordination Anion Modulation. Materials 2017, 10, 1360. https://doi.org/10.3390/ma10121360
Xiao L, Wu D, Wang X, Du W, Zhang J, Li S, Zhou H, Wu J, Tian Y. Four Novel Zn (II) Coordination Polymers Based on 4′-Ferrocenyl-3,2′:6′,3′′-Terpyridine: Engineering a Switch from 1D Helical Polymer Chain to 2D Network by Coordination Anion Modulation. Materials. 2017; 10(12):1360. https://doi.org/10.3390/ma10121360
Chicago/Turabian StyleXiao, Lufei, Dajun Wu, Xuchun Wang, Wei Du, Jun Zhang, Shengli Li, Hongping Zhou, Jieying Wu, and Yupeng Tian. 2017. "Four Novel Zn (II) Coordination Polymers Based on 4′-Ferrocenyl-3,2′:6′,3′′-Terpyridine: Engineering a Switch from 1D Helical Polymer Chain to 2D Network by Coordination Anion Modulation" Materials 10, no. 12: 1360. https://doi.org/10.3390/ma10121360
APA StyleXiao, L., Wu, D., Wang, X., Du, W., Zhang, J., Li, S., Zhou, H., Wu, J., & Tian, Y. (2017). Four Novel Zn (II) Coordination Polymers Based on 4′-Ferrocenyl-3,2′:6′,3′′-Terpyridine: Engineering a Switch from 1D Helical Polymer Chain to 2D Network by Coordination Anion Modulation. Materials, 10(12), 1360. https://doi.org/10.3390/ma10121360