Functionalization of Magnetic Chitosan Particles for the Sorption of U(VI), Cu(II) and Zn(II)—Hydrazide Derivative of Glycine-Grafted Chitosan
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Sorbents
2.2. pH Effect on Sorption Properties and the Approach of the Sorption Mechanism
2.3. Uptake Kinetics
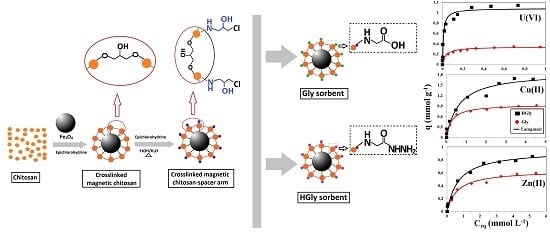
2.4. Sorption Isotherms
2.5. Sorption in Multi-Metal Solutions—Selectivity Study
2.6. Metal Desorption and Sorbent Recycling
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Sorbents
3.2.1. Synthesis of Magnetite-Chitosan Micro-Particles
3.2.2. Synthesis of Activated Magnetite-Chitosan Micro-Particles
3.2.3. Synthesis of Glycine Ester Hydrochloride
3.2.4. Synthesis of Gly Sorbent and Glycine-Ester Magnetic-Chitosan Particles
3.2.5. Synthesis of HGly
3.3. Characterization of Sorbents
3.4. Sorption Studies
3.5. Modeling of Uptake Kinetics and Sorption Isotherms
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, Z.; Xiao, Y.; Agterhuis, H.; Sietsma, J.; Yang, Y. Recycling of metals from urban mines—A strategic evaluation. J. Clean. Prod. 2016, 112, 2977–2987. [Google Scholar] [CrossRef]
- Biswas, S.; Rupawate, V.H.; Hareendran, K.N.; Roy, S.B.; Chakravartty, J.K. Novel precipitation technique for uranium recovery from carbonate leach solutions. J. Radioanal. Nucl. Chem. 2015, 304, 1345–1351. [Google Scholar] [CrossRef]
- Zhu, Z.W.; Pranolo, Y.; Cheng, C.Y. Uranium recovery from strong acidic solutions by solvent extraction with Cyanex 923 and a modifier. Miner. Eng. 2016, 89, 77–83. [Google Scholar] [CrossRef]
- Otrembska, P.; Gega, J. Separation of nickel(II) and cadmium(II) ions with ion-exchange and membrane processes. Sep. Sci. Technol. 2016, 51, 2675–2680. [Google Scholar] [CrossRef]
- Gando-Ferreira, L.M.; Romao, I.S.; Quina, M.J. Equilibrium and kinetic studies on removal of Cu2+ and Cr3+ from aqueous solutions using a chelating resin. Chem. Eng. J. 2011, 172, 277–286. [Google Scholar] [CrossRef]
- Atta, A.M.; Abdel-Rahman, A.A.H.; El Aassy, I.E.; Ahmed, F.Y.; Hamza, M.F. Adsorption properties of uranium (VI) ions on reactive crosslinked acrylamidoxime and acrylic acid copolymer resins. J. Dispersion Sci. Technol. 2011, 32, 84–94. [Google Scholar] [CrossRef]
- Rahmani-Sani, A.; Hosseini-Bandegharaei, A.; Hosseini, S.H.; Kharghani, K.; Zarei, H.; Rastegar, A. Kinetic, equilibrium and thermodynamic studies on sorption of uranium and thorium from aqueous solutions by a selective impregnated resin containing carminic acid. J. Hazard. Mater. 2015, 286, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Fialova, D.; Kremplova, M.; Melichar, L.; Kopel, P.; Hynek, D.; Adam, V.; Kizek, R. Interaction of heavy metal ions with carbon and iron based particles. Materials 2014, 7, 2242–2256. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Balasubramanian, R. Is biosorption suitable for decontamination of metal-bearing wastewaters? A critical review on the state-of-the-art of biosorption processes and future directions. J. Environ. Manag. 2015, 160, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Hackbarth, F.V.; Girardi, F.; Santos, J.C.; de Souza, A.A.U.; Boaventura, R.A.R.; de Souza, S.M.A.G.U.; Vilar, V.J.P. Ion-exchange breakthrough curves for single and multi-metal systems using marine macroalgae Pelvetia canaliculata as a natural cation exchanger. Chem. Eng. J. 2015, 269, 359–370. [Google Scholar] [CrossRef]
- He, J.; Chen, J.P. A comprehensive review on biosorption of heavy metals by algal biomass: Materials, performances, chemistry, and modeling simulation tools. Bioresour. Technol. 2014, 160, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, G.; Ceyhan, N.; Ozturk, T.; Akirmak, F.; Cosar, T. Biosorption of chromium(VI), cadmium(II) and copper(II) by Pantoea sp TEM. Chem. Eng. J. 2004, 102, 249–253. [Google Scholar] [CrossRef]
- Zhou, J.L. Zn biosorption by Rhizopus arrhizus and other fungi. Appl. Microbiol. Biotechnol. 1999, 51, 686–693. [Google Scholar] [CrossRef]
- Mata, Y.N.; Blazquez, M.L.; Ballester, A.; Gonzalez, F.; Munoz, J.A. Biosorption of cadmium, lead and copper with calcium alginate xerogels and immobilized Fucus vesiculosus. J. Hazard. Mater. 2009, 163, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Guibal, E. Interactions of metal ions with chitosan-based sorbents: A review. Sep. Purif. Technol. 2004, 38, 43–74. [Google Scholar] [CrossRef]
- Udoetok, I.A.; Wilson, L.D.; Headley, J.V. Quaternized cellulose hydrogels as sorbent materials and pickering emulsion stabilizing agents. Materials 2016, 9, 645. [Google Scholar] [CrossRef]
- Albernaz, V.L.; Joanitti, G.A.; Lopes, C.A.P.; Silva, L.P. Cellulose nanocrystals obtained from rice by products and their binding potential to metallic ions. J. Nanomater. 2015, 2015, 357384. [Google Scholar] [CrossRef]
- Kausar, A.; Bhatti, H.N.; MacKinnon, G. Equilibrium, kinetic and thermodynamic studies on the removal of U(VI) by low cost agricultural waste. Coll. Surf. B 2013, 111, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.A.; Dozol, J.F.; Nicotra, C.; Serose, J.; Massiani, C. Pyrolysis and incineration of cationic and anionic ion-exchange resins—Identification of volatile degradation compounds. J. Anal. Appl. Pyrolysis 1995, 31, 129–140. [Google Scholar] [CrossRef]
- Allouche, F.-N.; Guibal, E.; Mameri, N. Preparation of a new chitosan-based material and its application for mercury sorption. Coll. Surf. A 2014, 446, 224–232. [Google Scholar] [CrossRef]
- Guibal, E.; Vincent, T.; Navarro, R. Metal ion biosorption on chitosan for the synthesis of advanced materials. J. Mater. Sci. 2014, 49, 5505–5518. [Google Scholar] [CrossRef]
- Merrifield, J.D.; Davids, W.G.; MacRae, J.D.; Amirbahman, A. Uptake of mercury by thiol-grafted chitosan gel beads. Water Res. 2004, 38, 3132–3138. [Google Scholar] [CrossRef] [PubMed]
- Navarro, R.; Guzman, J.; Saucedo, I.; Revilla, J.; Guibal, E. Recovery of metal ions by chitosan: Sorption mechanisms and influence of metal speciation. Macromol. Biosci. 2003, 3, 552–561. [Google Scholar] [CrossRef]
- Elwakeel, K.Z.; Atia, A.A. Uptake of U(VI) from aqueous media by magnetic Schiff’s base chitosan composite. J. Clean. Prod. 2014, 70, 292–302. [Google Scholar] [CrossRef]
- Tan, L.; Zhang, X.; Liu, Q.; Jing, X.; Liu, J.; Song, D.; Hu, S.; Liu, L.; Wang, J. Synthesis of Fe3O4@TiO2 core-shell magnetic composites for highly efficient sorption of uranium (VI). Coll. Surf. A 2015, 469, 279–286. [Google Scholar] [CrossRef]
- Zhao, F.P.; Repo, E.; Sillanpaa, M.; Meng, Y.; Yin, D.L.; Tang, W.Z. Green synthesis of magnetic EDTA- and/or DTPA-cross-linked chitosan adsorbents for highly efficient removal of metals. Ind. Eng. Chem. Res. 2015, 54, 1271–1281. [Google Scholar] [CrossRef]
- Galhoum, A.A.; Mahfouz, M.G.; Atia, A.A.; Abdel-Rehem, S.T.; Gomaa, N.A.; Vincent, T.; Guibal, E. Amino acid functionalized chitosan magnetic nanobased particles for uranyl sorption. Ind. Eng. Chem. Res. 2015, 54, 12374–12385. [Google Scholar] [CrossRef]
- Mahfouz, M.G.; Galhoum, A.A.; Gomaa, N.A.; Abdel-Rehem, S.S.; Atia, A.A.; Vincent, T.; Guibal, E. Uranium extraction using magnetic nano-based particles of diethylenetriamine-functionalized chitosan: Equilibrium and kinetic studies. Chem. Eng. J. 2015, 262, 198–209. [Google Scholar] [CrossRef]
- Elkady, M.; Hassan, H.S.; Hashim, A. Immobilization of magnetic nanoparticles onto amine-modified nano-silica gel for copper ions remediation. Materials 2016, 9, 460. [Google Scholar] [CrossRef]
- Wang, K.; Qiu, G.M.; Cao, H.Y.; Jin, R.F. Removal of chromium(VI) from aqueous solutions using Fe3O4 magnetic polymer microspheres functionalized with amino groups. Materials 2015, 8, 8378–8391. [Google Scholar] [CrossRef]
- Oshita, K.; Takayanagi, T.; Oshima, M.; Motomizu, S. Adsorption behavior of cationic and anionic species on chitosan resins possessing amino acid moieties. Anal. Sci. 2007, 23, 1431–1434. [Google Scholar] [CrossRef] [PubMed]
- Galhoum, A.A.; Atia, A.A.; Mahfouz, M.G.; Abdel-Rehem, S.T.; Gomaa, N.A.; Vincent, T.; Guibal, E. Dy(III) recovery from dilute solutions using magnetic-chitosan nano-based particles grafted with amino acids. J. Mater. Sci. 2015, 50, 2832–2848. [Google Scholar] [CrossRef]
- Katarina, R.K.; Oshima, M.; Motomizu, S. High-capacity chitosan-based chelating resin for on-line collection of transition and rare-earth metals prior to inductively coupled plasma-atomic emission spectrometry measurement. Talanta 2009, 79, 1252–1259. [Google Scholar] [CrossRef] [PubMed]
- Albert, A. Quantitative studies of the avidity of naturally occurring substances for trace metals; amino-acids having only two ionizing groups. Biochem. J. 1950, 47, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Sorlier, P.; Denuzière, A.; Viton, C.; Domard, A. Relation between the degree of acetylation and the electrostatic properties of chitin and chitosan. Biomacromolecules 2001, 2, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Milletti, F.; Storchi, L.; Goracci, L.; Bendels, S.; Wagner, B.; Kansy, M.; Cruciani, G. Extending pKa prediction accuracy: High-throughput pKa measurements to understand pKa modulation of new chemical series. Eur. J. Med. Chem. 2010, 45, 4270–4279. [Google Scholar] [CrossRef] [PubMed]
- Hinman, R. Notes—Base strengths of some alkylhydrazines. J. Org. Chem. 1958, 23, 1587–1588. [Google Scholar] [CrossRef]
- Kumar, P.A.; Pisipati, V. Synthesis and characterization of novel metallomesogens: La(III), Pr(III) and Nd(III) complexes of N-(2-hydroxy-4-n-alkoxy-benzaldehydeimino)-2-benzamidoethanamide. Synth. React. Inorg. Met.-Org. Chem. 2000, 30, 1099–1112. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Siafaka, P.I.; Pavlidou, E.G.; Chrissafis, K.J.; Bikiaris, D.N. Synthesis and adsorption application of succinyl-grafted chitosan for the simultaneous removal of zinc and cationic dye from binary hazardous mixtures. Chem. Eng. J. 2015, 259, 438–448. [Google Scholar] [CrossRef]
- Tan, Z.Y.; Peng, H.; Liu, H.F.; Wang, L.L.; Chen, J.; Lu, X.H. Facile preparation of EDTA-functionalized chitosan magnetic adsorbent for removal of Pb(II). J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Guzman, J.; Saucedo, I.; Revilla, J.; Navarro, R.; Guibal, E. Copper sorption by chitosan in the presence of citrate ions: Influence of metal speciation on sorption mechanism and uptake capacities. Int. J. Biol. Macromol. 2003, 33, 57–65. [Google Scholar] [CrossRef]
- Včeláková, K.; Zusková, I.; Kenndler, E.; Gaš, B. Determination of cationic mobilities and pKa values of 22 amino acids by capillary zone electrophoresis. Electrophoresis 2004, 25, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Lindegren, C.R.; Niemann, C. The apparent ionization constants of acethydrazide and glycylhydrazide. J. Am. Chem. Soc. 1949, 71, 1504. [Google Scholar] [CrossRef]
- Feitoza, N.C.; Goncalves, T.D.; Mesquita, J.J.; Menegucci, J.S.; Santos, M.; Chaker, J.A.; Cunha, R.B.; Medeiros, A.M.M.; Rubim, J.C.; Sousa, M.H. Fabrication of glycine-functionalized maghemite nanoparticles for magnetic removal of copper from wastewater. J. Hazard. Mater. 2014, 264, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Guibal, E.; Milot, C.; Roussy, J. Influence of hydrolysis mechanisms on molybdate sorption isotherms using chitosan. Sep. Sci. Technol. 2000, 35, 1021–1038. [Google Scholar] [CrossRef]
- Guzman, J.; Saucedo, I.; Navarro, R.; Revilla, J.; Guibal, E. Vanadium interactions with chitosan: Influence of polymer protonation and metal speciation. Langmuir 2002, 18, 1567–1573. [Google Scholar] [CrossRef]
- Baes, C.F., Jr.; Mesmer, R.E. Hydrolysis of Cations; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Guibal, E.; Roulph, C.; Lecloirec, P. Uranium biosorption by a filamentous fungus Mucor miehei: pH effect on mechanisms and performances of uptake. Water Res. 1992, 26, 1139–1145. [Google Scholar] [CrossRef]
- Su, J.; Zhang, K.; Schwarz, W.H.E.; Li, J. Uranyl-glycine-water complexes in solution: Comprehensive computational modeling of coordination geometries, stabilization energies, and luminescence properties. Inorg. Chem. 2011, 50, 2082–2093. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.Y.; Lau, R.J.; Yang, K.L. Preparation of ion-imprinted silica gels functionalized with glycine, diglycine, and triglycine and their adsorption properties for copper ions. Langmuir 2007, 23, 8079–8086. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.G. Acids and bases. Science 1966, 151, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.L. Hard and soft acids bases (HSAB) principle and organic-chemistry. Chem. Rev. 1975, 75, 1–20. [Google Scholar] [CrossRef]
- Atalay, Y.B.; Di Toro, D.M.; Carbonaro, R.F. Estimation of stability constants for metal-ligand complexes containing neutral nitrogen donor atoms with applications to natural organic matter. Geochim. Cosmochim. Acta 2013, 122, 464–477. [Google Scholar] [CrossRef]
- Wang, W.B.; Huang, D.J.; Kang, Y.R.; Wang, A.Q. One-step in situ fabrication of a granular semi-IPN hydrogel based on chitosan and gelatin for fast and efficient adsorption of Cu2+ ion. Coll. Surf. B 2013, 106, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Hamza, M.F.; Abdel-Rahman, A.A.H. Extraction studies of some hazardous metal ions using magnetic peptide resins. J. Dispersion Sci. Technol. 2015, 36, 411–422. [Google Scholar] [CrossRef]
- Galhoum, A.A.; Mahfouz, M.G.; Gomaa, N.A.; Abdel-Rehem, S.S.; Atia, A.A.; Vincent, T.; Guibal, E. Cysteine-functionalized chitosan magnetic nano-based particles for the recovery of uranium(VI): Uptake kinetics and sorption isotherms. Sep. Sci. Technol. 2015, 50, 2776–2789. [Google Scholar]
- Lin, W.; Carboni, M.; Abney, C.W.; Taylor-Pashow, K.M.L.; Vivero-Escoto, J.L. Uranium sorption with functionalized mesoporous carbon materials. Ind. Eng. Chem. Res. 2013, 52, 15187–15197. [Google Scholar]
- Cao, Q.; Liu, Y.; Kong, X.; Zhou, L.; Guo, H. Synthesis of phosphorus-modified poly(styrene-co-divinylbenzene) chelating resin and its adsorption properties of uranium(VI). J. Radioanal. Nucl. Chem. 2013, 298, 1137–1147. [Google Scholar] [CrossRef]
- Wang, G.H.; Liu, J.S.; Wang, X.G.; Xie, Z.Y.; Deng, N.S. Adsorption of uranium (VI) from aqueous solution onto cross-linked chitosan. J. Hazard. Mater. 2009, 168, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.K.; Handa, A.; Sait, S.S.; Shrivastav, P.; Agrawal, Y.K. Pre-concentration, separation and trace determination of lanthanum(III), cerium(III), thorium(IV) and uranium(VI) on polymer supported o-vanillinsemicarbazone. Anal. Chim. Acta 2001, 429, 237–246. [Google Scholar] [CrossRef]
- Zhou, L.; Shang, C.; Liu, Z.; Huang, G.; Adesina, A.A. Selective adsorption of uranium(VI) from aqueous solutions using the ion-imprinted magnetic chitosan resins. J. Colloid Interface Sci. 2012, 366, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Birlik, E.; Ersoz, A.; Denizli, A.; Say, R. Preconcentration of copper using double-imprinted polymer via solid phase extraction. Anal. Chim. Acta 2006, 565, 145–151. [Google Scholar] [CrossRef]
- Huang, S.H.; Chen, D.H. Rapid removal of heavy metal cations and anions from aqueous solutions by an amino-functionalized magnetic nano-adsorbent. J. Hazard. Mater. 2009, 163, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Mandavian, A.R.; Mirrahimi, M.A.S. Efficient separation of heavy metal cations by anchoring polyacrylic acid on superparamagnetic magnetite nanoparticles through surface modification. Chem. Eng. J. 2010, 159, 264–271. [Google Scholar] [CrossRef]
- Shukla, S.R.; Pai, R.S. Adsorption of Cu(II), Ni(II) and Zn(II) on dye loaded groundnut shells and sawdust. Sep. Purif. Technol. 2005, 43, 1–8. [Google Scholar] [CrossRef]
- Wong, K.K.; Lee, C.K.; Low, K.S.; Haron, M.J. Removal of Cu and Pb from electroplating wastewater using tartaric acid modified rice husk. Proc. Biochem. 2003, 39, 437–445. [Google Scholar] [CrossRef]
- Ngah, W.S.W.; Teong, L.C.; Toh, R.H.; Hanafiah, M. Comparative study on adsorption and desorption of Cu(II) ions by three types of chitosan-zeolite composites. Chem. Eng. J. 2013, 223, 231–238. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Hu, J.; Wang, J.L. Competitive adsorption of Pb(II), Cu(II) and Zn(II) onto xanthate-modified magnetic chitosan. J. Hazard. Mater. 2012, 221, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.M.; Wang, Y.P.; Liu, Z.R.; Huang, Q.W. Characteristics of equilibrium, kinetics studies for adsorption of Hg(II), Cu(II), and Ni(II) ions by thiourea-modified magnetic chitosan microspheres. J. Hazard. Mater. 2009, 161, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.T.; Nie, H.L.; Branford-White, C.; He, Z.Y.; Zhu, L.M. Removal of Cu2+ from aqueous solution by chitosan-coated magnetic nanoparticles modified with alpha-ketoglutaric acid. J. Coll. Interface Sci. 2009, 330, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.W.; Kan, C.C.; Rogel, B.D.; Dalida, M.L.P. Adsorption of copper (II) and lead (II) ions from aqueous solution on chitosan-coated sand. Carbohydr. Polym. 2010, 80, 891–899. [Google Scholar] [CrossRef]
- Jayakumar, R.; Rajkumar, M.; Freitas, H.; Selvamurugan, N.; Nair, S.V.; Furuike, T.; Tamura, H. Preparation, characterization, bioactive and metal uptake studies of alginate/phosphorylated chitin blend films. Int. J. Biol. Macromol. 2009, 44, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Ngah, W.S.W.; Kamari, A.; Koay, Y. Equilibrium and kinetics studies of adsorption of copper(II) on chitosan and chitosan/PVA beads. Int. J. Biol. Macromol. 2004, 34, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Popuri, S.R.; Vijaya, Y.; Boddu, V.M.; Abburi, K. Adsorptive removal of copper and nickel ions from water using chitosan coated PVC beads. Bioresour. Technol. 2009, 100, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.P.; Chung, Y.C.; Liou, M.R. Adsorption of Cu(II) and Ni(II) by pelletized biopolymer. J. Hazard. Mater. 1996, 45, 265–277. [Google Scholar] [CrossRef]
- Cheung, W.H.; Ng, J.C.Y.; McKay, G. Kinetic analysis of the sorption of copper(II) ions on chitosan. J. Chem. Technol. Biotechnol. 2003, 78, 562–571. [Google Scholar] [CrossRef]
- Schmuhl, R.; Krieg, H.M.; Keizer, K. Adsorption of Cu(II) and Cr(VI) ions by chitosan: Kinetics and equilibrium studies. Water SA 2001, 27, 1–7. [Google Scholar] [CrossRef]
- Tan, S.Y.; Wang, Y.T.; Peng, C.H.; Tang, Y.R. Synthesis and adsorption properties for metal ions of crosslinked chitosan acetate crown ethers. J. Appl. Polym. Sci. 1999, 71, 2069–2074. [Google Scholar] [CrossRef]
- Futalan, C.M.; Kan, C.C.; Dalida, M.L.; Hsien, K.J.; Pascua, C.; Wan, M.W. Comparative and competitive adsorption of copper, lead, and nickel using chitosan immobilized on bentonite. Carbohydr. Polym. 2011, 83, 528–536. [Google Scholar] [CrossRef]
- Chen, Y.W.; Wang, J.L. Preparation and characterization of magnetic chitosan nanoparticles and its application for Cu(II) removal. Chem. Eng. J. 2011, 168, 286–292. [Google Scholar]
- Hokkanen, S.; Repo, E.; Sillanpaa, M. Removal of heavy metals from aqueous solutions by succinic anhydride modified mercerized nanocellulose. Chem. Eng. J. 2013, 223, 40–47. [Google Scholar] [CrossRef]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1249. [Google Scholar] [CrossRef]





| Material | C (%) | H (%) | N (%) |
|---|---|---|---|
| Activated magnetic chitosan | 15.43 (±0.33) | 2.61 (±0.15) | 1.79 (±0.03) |
| Gly sorbent | 19.41 (±0.35) | 2.82 (±0.01) | 4.46 (±0.03) |
| Esterified Gly sorbent | 27.95 (±0.04) | 2.95 (±0.04) | 4.81 (±0.05) |
| HGly | 23.74 (±0.61) | 4.03 (±0.12) | 10.05 (±0.07) |
| Sorbent | Metal | qeq,exp | qeq,calc. | k1 × 102 | EV × 104 | qeq,calc. | k2 × 101 | EV × 104 |
|---|---|---|---|---|---|---|---|---|
| PFORE | PSORE | |||||||
| Gly | U(VI) | 0.166 | 0.157 | 1.73 | 1.44 | 0.171 | 1.28 | 1.12 |
| Cu(II) | 0.429 | 0.295 | 6.26 | 41.2 | 0.318 | 2.36 | 27.9 | |
| Zn(II) | 0.192 | 0.169 | 32.4 | 4.93 | 0.175 | 26.2 | 2.58 | |
| HGly | U(VI) | 0.404 | 0.363 | 10.8 | 16.2 | 0.386 | 4.01 | 6.41 |
| Cu(II) | 0.842 | 0.666 | 13.0 | 104.2 | 0.701 | 2.41 | 58.3 | |
| Zn(II) | 0.375 | 0.363 | 11.7 | 7.38 | 0.384 | 1.17 | 5.84 | |
| Sorbent | Metal | qm,exp | qm,L | bL | EV × 103 | qm,S | bS | n | EV × 103 |
|---|---|---|---|---|---|---|---|---|---|
| Langmuir Equation | Sips Equation | ||||||||
| Gly | U(VI) | 0.349 | 0.338 | 32.8 | 0.674 | 0.474 | 2.98 | 2.14 | 0.064 |
| Cu(II) | 0.920 | 0.946 | 3.78 | 1.305 | 1.03 | 2.33 | 1.29 | 1.16 | |
| Zn(II) | 0.598 | 0.641 | 1.59 | 1.95 | 1.23 | 0.418 | 2.01 | 1.09 | |
| HGly | U(VI) | 1.14 | 1.082 | 106.9 | 70.9 | 1.32 | 7.86 | 1.93 | 1.41 |
| Cu(II) | 1.69 | 1.878 | 1.56 | 16.1 | 10.2 | 0.114 | 2.52 | 9.29 | |
| Zn(II) | 0.853 | 0.952 | 1.40 | 1.35 | 1.03 | 1.13 | 1.16 | 1.67 | |
| Sorbent | pH | qm (mg·g−1) | Ref. | ||
|---|---|---|---|---|---|
| U(VI) | Cu(II) | Zn(II) | |||
| Cysteine-chitosan magnetic nano-based particles | 4 | 101 | [56] | ||
| Alanine-chitosan magnetic nano-based particles | 4 | 88 | [27] | ||
| Functionalized mesoporous carbon | 4 | 97 | [57] | ||
| Phosphorus-modified resin | 5 | 89 | [58] | ||
| Cross-linked chitosan | 3 | 74.0 | [59] | ||
| Merrifield chloromethylated resin anchored with semicarbazone moiety | 6.5 | 48.7 | [60] | ||
| Ion-imprinted magnetic chitosan resins (IMCR) -glutaraldehyde | 5 | 187.3 | [61] | ||
| Chitosan-succinate (CS) imprinted polymers | 7 | 47.6 | [62] | ||
| Amino-modified Fe3O4 | 5.2 | 12.4 | [63] | ||
| m-PAA-Na-coated MNPs (magnetic nanoparticles) | 8 | 30.0 | [64] | ||
| Sawdust | 5.2 | 8.1 | [65] | ||
| Rice husk | 5.2 | 31.9 | [66] | ||
| Chitosan–zeolite composites | 5 | 14.8 | [67] | ||
| Semi-IPN hydrogel based on chitosan and gelatin | 5.5 | 153.9 | [54] | ||
| Xanthate-modified magnetic chitosan | 5 | 34.5 | [68] | ||
| Thiourea-modified magnetic chitosan microspheres | 5 | 66.7 | [69] | ||
| α-ketoglutaric acid modified chitosan–coated magnetic nanoparticles Cu(II) | 6 | 96.2 | [70] | ||
| chitosan-coated sand | 3 | 8.4 | [71] | ||
| Alginate/phosphorylated chitin blend film | 5 | 11.70 | [72] | ||
| Chitosan/PVA (polyvinyl alcohol) | 6 | 47.9 | [73] | ||
| Chitosan coated PVC (polyvinyl chloride) | 4 | 87.9 | [74] | ||
| Chitosan | 5 | 16.8 | [75] | ||
| Chitosan | 4.5 | 71.2 | [76] | ||
| Non-cross linked chitosan | 5 | 80 | [77] | ||
| Chitosan acetate crown ether (CCTS–1) | 5.6 | 23 | [78] | ||
| Chitosan immobilized bentonite (CHB) | 4 | 20 | [79] | ||
| Chitosan-magnetite nanocomposites | 5 | 35.5 | [80] | ||
| Succinic anhydride-modified mercerized nanocellulose | 5 | 105.3 | [81] | ||
| Magnetic glycine-peptide | 5 | 455 | [55] | ||
| Gly sorbent | 5 | 80.3 | 57.6 | 23.5 | This work |
| HGly sorbent | 5 | 186.5 | 110.3 | 46.4 | This work |
| Metal | Sorbent | Cycle #1 | Cycle #2 | Cycle #3 | Cycle #4 | Cycle #5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S | D | S | D | S | D | S | D | S | D | ||
| U(VI) | Gly | 97.6 | 91.0 | 96.3 | 97.9 | 96.1 | 98.2 | 92.5 | 98.5 | 88.9 | 97.7 (±3.6) |
| (±1.8) | (±4.7) | (±0.1) | (±0.9) | (±0.2) | (±0.6) | (±0.2) | (±2.6) | (±0.1) | |||
| HGly | 97.8 | 95.5 | 97.1 | 98.8 | 94.9 | 97.0 | 92.2 | 95.6 | 92.2 | 95.7 | |
| (±2.1) | (±2.6) | (±1.6) | (±2.8) | (±0.3) | (±1.0) | (±0.1) | (±1.1) | (±0.4) | (±1.8) | ||
| Cu(II) | Gly | 57.7 | 98.7 | 54.8 | 98.0 | 55.4 | 93.6 | 53.8 | 95.0 | 51.1 | 93.2 |
| (±2.1) | (±0.2) | (±1.1) | (±2.2) | (±0.3) | (±0.1) | (±2.0) | (±6.4) | (±1.2) | (±3.4) | ||
| HGly | 57.5 | 96.8 | 55.3 | 98.8 | 53.6 | 98.4 | 48.7 | 98.0 | 46.1 | 94.4 | |
| (±1.6) | (±0.8) | (±0.4) | (±0.4) | (±0.1) | (±0.9) | (±3.0) | (±1.0) | (±0.3) | (±2.0) | ||
| Zn(II) | Gly | 70.3 | 87.1 | 69.2 | 86.8 | 67.9 | 87.2 | 66.1 | 90.5 | 63.7 | 93.2 |
| (±1.1) | (± 0.1) | (±0.9) | (± 0.8) | (±0.3) | (±2.9) | (±1.3) | (±0.1) | (±1.8) | (±3.4) | ||
| HGly | 99.9 | 98.8 | 95.2 | 100.2 | 92.9 | 96.8 | 92.2 | 93.3 | 91.0 | 93.1 | |
| (±0.1) | (±1.1) | (±0.1) | (±1.8) | (±1.1) | (±1.8) | (±0.1) | (±1.0) | (±0.4) | (±0.6) | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamza, M.F.; Aly, M.M.; Abdel-Rahman, A.A.-H.; Ramadan, S.; Raslan, H.; Wang, S.; Vincent, T.; Guibal, E. Functionalization of Magnetic Chitosan Particles for the Sorption of U(VI), Cu(II) and Zn(II)—Hydrazide Derivative of Glycine-Grafted Chitosan. Materials 2017, 10, 539. https://doi.org/10.3390/ma10050539
Hamza MF, Aly MM, Abdel-Rahman AA-H, Ramadan S, Raslan H, Wang S, Vincent T, Guibal E. Functionalization of Magnetic Chitosan Particles for the Sorption of U(VI), Cu(II) and Zn(II)—Hydrazide Derivative of Glycine-Grafted Chitosan. Materials. 2017; 10(5):539. https://doi.org/10.3390/ma10050539
Chicago/Turabian StyleHamza, Mohammed F., Mohsen M. Aly, Adel A.-H. Abdel-Rahman, Samar Ramadan, Heba Raslan, Shengye Wang, Thierry Vincent, and Eric Guibal. 2017. "Functionalization of Magnetic Chitosan Particles for the Sorption of U(VI), Cu(II) and Zn(II)—Hydrazide Derivative of Glycine-Grafted Chitosan" Materials 10, no. 5: 539. https://doi.org/10.3390/ma10050539
APA StyleHamza, M. F., Aly, M. M., Abdel-Rahman, A. A. -H., Ramadan, S., Raslan, H., Wang, S., Vincent, T., & Guibal, E. (2017). Functionalization of Magnetic Chitosan Particles for the Sorption of U(VI), Cu(II) and Zn(II)—Hydrazide Derivative of Glycine-Grafted Chitosan. Materials, 10(5), 539. https://doi.org/10.3390/ma10050539