Simultaneously Toughening and Strengthening Soy Protein Isolate-Based Composites via Carboxymethylated Chitosan and Halloysite Nanotube Hybridization
Abstract
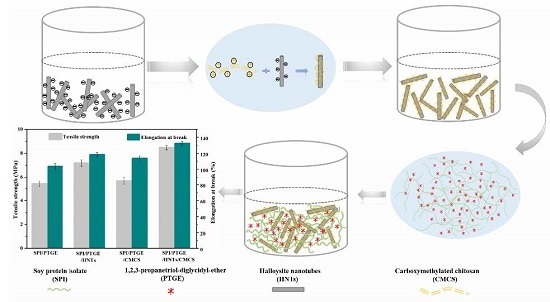
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Carboxymethylated Chitosan/Halloysite Nanotubes Hybrid
2.3. Soy Protein Isolate Film Preparation
2.4. Characterization
2.4.1. Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.4.2. X-ray Photoelectron Spectroscopy
2.4.3. X-ray Diffraction
2.4.4. Scanning Electron Microscopy
2.4.5. Atomic Force Microscopy
2.4.6. Opacity
2.4.7. Water Absorption and Total Soluble Matter
2.4.8. Contact Angle Determination
2.4.9. Mechanical Properties and Coating Thickness
2.4.10. Statistical Analysis
3. Results and Discussion
3.1. Structural Analysis of the Soy Protein Isolate-Based Films
3.2. Soy Protein Isolate Film Micromorphology
3.3. Opacity
3.4. Mechanical Properties of the Composite Films
3.5. Water Resistance and Surface Hydrophilicity Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- González, A.; Alvarez Igarzabal, C.I. Nanocrystal-reinforced soy protein films and their application as active packaging. Food Hydrocoll. 2015, 43, 777–784. [Google Scholar] [CrossRef]
- Gupta, P.; Nayak, K.K. Characteristics of protein-based biopolymer and its application. Polym. Eng. Sci. 2015, 55, 485–498. [Google Scholar] [CrossRef]
- Kang, H.; Song, X.; Wang, Z.; Zhang, W.; Zhang, S.; Li, J. High-Performance and Fully Renewable Soy Protein Isolate-Based Film from Microcrystalline Cellulose via Bio-Inspired Poly(dopamine) Surface Modification. ACS Sustain. Chem. Eng. 2016, 4, 4354–4360. [Google Scholar] [CrossRef]
- Li, K.; Chen, H.; Li, Y.; Li, J.; He, J. Endogenous Cu and Zn nanocluster-regulated soy protein isolate films: Excellent hydrophobicity and flexibility. RSC Adv. 2015, 5, 66543–66548. [Google Scholar] [CrossRef]
- Li, W.; Zhao, H.; He, Z.; Zeng, M.; Qin, F.; Chen, J. Modification of soy protein hydrolysates by Maillard reaction: Effects of carbohydrate chain length on structural and interfacial properties. Colloids Surf. 2016, 138, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Nandane, A.S.; Jain, R. Study of mechanical properties of soy protein based edible film as affected by its composition and process parameters by using RSM. Int. J. Food Sci. Technol. 2015, 52, 3645–3650. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Yang, Y.; Yao, J.; Shao, Z.; Chen, X. Robust soy protein films obtained by slight chemical modification of polypeptide chains. Polym. Chem. 2013, 4, 5425–5431. [Google Scholar] [CrossRef]
- Klüver, E.; Meyer, M. Thermoplastic processing, rheology, and extrudate properties of wheat, soy, and pea proteins. Polym. Eng. Sci. 2015, 55, 1912–1919. [Google Scholar] [CrossRef]
- Li, C.; Luo, J.; Qin, Z.; Chen, H.; Gao, Q.; Li, J. Mechanical and thermal properties of microcrystalline cellulose-reinforced soy protein isolate–gelatin eco-friendly films. RSC Adv. 2015, 5, 56518–56525. [Google Scholar] [CrossRef]
- Xia, C.; Zhang, S.; Shi, S.Q.; Cai, L.; Garcia, A.C.; Rizvi, H.R.; D’Souza, N.A. Property enhancement of soy protein isolate-based films by introducing POSS. Int. J. Biol. Macromol. 2016, 82, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhang, W.; Zhang, S.; Li, L.; Li, J.; Zhang, Y. Preparation and characterization of poly(vinyl alcohol) and 1,2,3-propanetriol diglycidyl ether incorporated soy proteinisolate-based films. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Liu, X.; Song, R.; Zhang, W.; Qi, C.; Zhang, S.; Li, J. Development of Eco-friendly Soy Protein Isolate Films with High Mechanical Properties through HNTs, PVA, and PTGE Synergism Effect. Sci. Rep. 2017, 7, 44289. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, Q.; Wu, S.; Gou, Z.; Wu, X.; Xu, D. Starch/chitosan films reinforced with polydopamine modified MMT: Effects of dopamine concentration. Food Hydrocoll. 2016, 61, 678–684. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Dong, Y.; Li, K.; Li, L.; Li, J. Carbon nanoparticles/soy protein isolate bio-films with excellent mechanical and water barrier properties. Ind. Crops Prod. 2016, 82, 133–140. [Google Scholar] [CrossRef]
- Ai, F.; Zheng, H.; Wei, M.; Huang, J. Soy protein plastics reinforced and toughened by SiO2 nanoparticles. J. Appl. Polym. Sci. 2007, 105, 1597–1604. [Google Scholar] [CrossRef]
- Dash, S.; Kisku, S.K.; Swain, S.K. Effect of nanoclay on morphological, thermal, and barrier properties of albumin bovine. Polym Compos. 2012, 33, 2201–2206. [Google Scholar] [CrossRef]
- Gonzalez, A.; Tartara, L.I.; Palma, S.D.; Alvarez Igarzabal, C.I. Crosslinked soy protein films and their application as ophthalmic drug delivery system. Mat. Sci. Eng. C Mater. 2015, 51, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Sandeep, K.P.; Alavi, S.; Truong, V.D.; Gorga, R.E. Preparation and characterization of bio-nanocomposite films based on soy protein isolate and montmorillonite using melt extrusion. J. Food Eng. 2010, 100, 480–489. [Google Scholar] [CrossRef]
- De Silva, R.T.; Pasbakhsh, P.; Goh, K.L.; Chai, S.-P.; Ismail, H. Physico-chemical characterisation of chitosan/halloysite composite membranes. Polym. Test. 2013, 32, 265–271. [Google Scholar] [CrossRef]
- Hebbar, R.S.; Isloor, A.M.; Ananda, K.; Ismail, A.F. Fabrication of polydopamine functionalized halloysite nanotube/polyetherimide membranes for heavy metal removal. J. Mater. Chem. 2016, 4, 764–774. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Palmisano, G.; Parisi, F. Halloysite nanotube with fluorinated lumen: Non-foaming nanocontainer for storage and controlled release of oxygen in aqueous media. J. Colloid Interface Sci. 2014, 417, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Guo, B.; Du, M.; Lei, Y.; Jia, D. Natural inorganic nanotubes reinforced epoxy resin nanocomposites. J. Polym. Res. 2007, 15, 205–212. [Google Scholar] [CrossRef]
- Luo, C.; Zou, Z.; Luo, B.; Wen, W.; Li, H.; Liu, M.; Zhou, C. Enhanced mechanical properties and cytocompatibility of electrospun poly(l-lactide) composite fiber membranes assisted by polydopamine-coated halloysite nanotubes. Appl. Surf. Sci. 2016, 369, 82–91. [Google Scholar] [CrossRef]
- Kubade, P.; Tambe, P. Influence of surface modification of halloysite nanotubes and its localization in PP phase on mechanical and thermal properties of PP/ABS blends. Compos. Interface 2017, 24, 469–487. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Hydrophobically Modified Halloysite Nanotubes as Reverse Micelles for Water-in-Oil Emulsion. Langmuir 2015, 31, 7472–7478. [Google Scholar] [CrossRef] [PubMed]
- Lvov, Y.; Wang, W.; Zhang, L.; Fakhrullin, R. Halloysite Clay Nanotubes for Loading and Sustained Release of Functional Compounds. Adv. Mater. 2016, 28, 1227–1250. [Google Scholar] [CrossRef] [PubMed]
- Gorrasi, G.; Senatore, V.; Vigliotta, G.; Belviso, S.; Pucciariello, R. PET—halloysite nanotubes composites for packaging application: Preparation, characterization and analysis of physical properties. Eur. Polym. J. 2014, 61, 145–156. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Salvetat, J.-P.; Saboungi, M.-L. Reinforcement of semicrystalline polymers with collagen-modified single walled carbon nanotubes. Appl. Phys. Lett. 2006, 88, 233119. [Google Scholar] [CrossRef]
- Dumont, V.C.; Mansur, A.A.; Carvalho, S.M.; Medeiros Borsagli, F.G.; Pereira, M.M.; Mansur, H.S. Chitosan and carboxymethyl-chitosan capping ligands: Effects on the nucleation and growth of hydroxyapatite nanoparticles for producing biocomposite membranes. Mater. Sci. Eng. 2016, 59, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Vo, D.T.; Sabrina, S.; Lee, C.K. Silver deposited carboxymethyl chitosan-grafted magnetic nanoparticles as dual action deliverable antimicrobial materials. Mater. Sci. Eng. 2017, 73, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Kang, H.; Zhang, W.; Zhang, S.; Li, J. Improvement of Interfacial Adhesion by Bio-Inspired Catechol-Functionalized Soy Protein with Versatile Reactivity: Preparation of Fully Utilizable Soy-Based Film. Polymers 2017, 9, 95. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhang, S.; Shi, J. Preparation and Characterization of All-Biomass SPI-Based Films Enhanced by Epoxy Castor Oil Acid Sodium and Hydroxypropyl Cellulose. Materials 2016, 9, 193. [Google Scholar] [CrossRef]
- Zhang, S.; Xia, C.; Dong, Y.; Yan, Y.; Li, J.; Shi, S.Q.; Cai, L. SPI-based films reinforced by surface modified cellulose nanocrystal. Ind. Crop Prod. 2016, 80, 207–213. [Google Scholar] [CrossRef]
- Dong, Y.; Marshall, J.; Haroosh, H.J.; Mohammadzadehmoghadam, S.; Liu, D.; Qi, X.; Lau, K.-T. Polylactic acid (PLA)/halloysite nanotube (HNT) composite mats: Influence of HNT content and modification. Compos. Part A Appl. Sci. Manuf. 2015, 76, 28–36. [Google Scholar] [CrossRef]
- Chen, N.; Lin, Q.; Rao, J.; Zeng, Q. Water resistances and bonding strengths of soy-based adhesives containing different carbohydrates. Ind. Crop Prod. 2013, 50, 44–49. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.; Li, X.; Li, K.; Gao, Q.; Li, J. Toughening improvement to a soybean meal-based bioadhesive using an interpenetrating acrylic emulsion network. J. Mater. Sci. 2016, 51, 9330–9341. [Google Scholar] [CrossRef]
- Liu, M.; Li, W.; Rong, J.; Zhou, C. Novel polymer nanocomposite hydrogel with natural clay nanotubes. Colloid Polym. Sci. 2012, 290, 895–905. [Google Scholar] [CrossRef]
- Dong, Y.; Bickford, T.; Haroosh, H.J.; Lau, K.-T.; Takagi, H. Multi-response analysis in the material characterisation of electrospun poly (lactic acid)/halloysite nanotube composite fibres based on Taguchi design of experiments: Fibre diameter, non-intercalation and nucleation effects. Appl. Phys. 2013, 112, 747–757. [Google Scholar] [CrossRef]
- Gong, B.; Ouyang, C.; Gao, Q. Synthesis and properties of a millable polyurethane nanocomposite based on castor oil and halloysite nanotubes. RSC Adv. 2016, 6, 12084–12092. [Google Scholar] [CrossRef]
- Liu, M.; Wu, C.; Jiao, Y.; Xiong, S.; Zhou, C. Chitosan–halloysite nanotubes nanocomposite scaffolds for tissue engineering. J. Mater. Chem. 2013, 1, 2078–2089. [Google Scholar] [CrossRef]
- Zheng, T.; Yu, X.; Pilla, S. Mechanical and moisture sensitivity of fully bio-based dialdehyde carboxymethyl cellulose cross-linked SPI films. Carbohyd. Polym. 2017, 157, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, D.; Zhang, J. Novel Polyvinyl Alcohol/Styrene Butadiene Rubber Latex/Carboxymethyl Cellulose Nanocomposites Reinforced with Modified Halloysite Nanotubes. J. Nanomater. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Tham, W.L.; Poh, B.T.; Mohd Ishak, Z.A.; Chow, W.S. Transparent poly(lactic acid)/halloysite nanotube nanocomposites with improved oxygen barrier and antioxidant properties. J. Therm. Anal. Calorim. 2016, 126, 1331–1337. [Google Scholar] [CrossRef]
- Lvov, Y.; Abdullayev, E. Functional polymer-clay nanotube composites with sustained release of chemical agents. Prog. Polym. Sci. 2013, 38, 1690–1719. [Google Scholar] [CrossRef]
- Khoo, W.S.; Ismail, H.; Ariffin, A. Tensile, Swelling, and Oxidative Degradation Properties of Crosslinked Polyvinyl Alcohol/Chitosan/Halloysite Nanotube Composites. Int. J. Polym. Mater. 2013, 62, 390–396. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, H.; Wu, J.; Ye, L. High impact strength epoxy nanocomposites with natural nanotubes. Polymer 2007, 48, 6426–6433. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, C.; Liu, M.; Yang, Z.; Tjiu, W.W.; Liu, T. Simultaneous reinforcement and toughening of polyurethane composites with carbon nanotube/halloysite nanotube hybrids. Compos. Sci. Technol. 2014, 91, 98–103. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Suriyatem, R. Moisture sorption isotherms of SPI/carboxymethyl chitosan blend films. J. Agric. Sci. Technol. 2012, 2, 50. [Google Scholar]








| Sample | SPI (g) | Glycerol (g) | Water (g) | PTGE (g) | HNTs (g) | CMCS (g) |
|---|---|---|---|---|---|---|
| a | 3 | 1.5 | 57 | 0.3 | 0 | 0 |
| b | 3 | 1.5 | 57 | 0.3 | 0.09 | 0 |
| c | 3 | 1.5 | 57 | 0.3 | 0 | 0.09 |
| d | 3 | 1.5 | 57 | 0.3 | 0.09 | 0.09 |
| Sample | C1 (%) | C2 (%) | C3 (%) | C4 (%) | C5 (%) |
|---|---|---|---|---|---|
| a | 64.42 | 6.67 | 19.88 | 9.66 | 1.37 |
| b | 63.06 | 4.45 | 20.00 | 10.76 | 1.73 |
| c | 66.43 | 5.59 | 17.70 | 9.46 | 0.82 |
| d | 68.26 | 7.09 | 13.55 | 11.09 | 0 |
| Sample | Thickness (mm) Mean (SD) | Tensile Strength (MPa) Mean (SD) | Elongation at Break (%) Mean (SD) |
|---|---|---|---|
| a | 0.26 (0.013) | 5.39 (0.22) | 103.75 (3.82) |
| b | 0.25 (0.011) | 7.16 (0.27) | 118.10 (2.64) |
| c | 0.20 (0.015) | 5.68 (0.31) | 113.42 (3.38) |
| d | 0.23 (0.009) | 8.47 (0.19) | 132.12 (2.75) |
| Increment (%) a | - | 57.14 | 27.34 |
| Sample | Water Absorption (%) Mean (SD) | Total Soluble Matter (%) Mean (SD) | Water Contact Angles (°) Mean (SD) |
|---|---|---|---|
| a | 64.10 (1.78) | 31.27 (0.98) | 34.83 (2.24) |
| b | 63.02 (1.26) | 29.06 (1.45) | 36.23 (1.97) |
| c | 60.50 (2.01) | 30.14 (1.36) | 31.83 (0.87) |
| d | 61.34 (1.15) | 29.98 (1.77) | 38.96 (1.42) |
| Increment (%) a | −4.31 | −4.13 | 11.86 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Kang, H.; Wang, Z.; Zhang, W.; Li, J.; Zhang, S. Simultaneously Toughening and Strengthening Soy Protein Isolate-Based Composites via Carboxymethylated Chitosan and Halloysite Nanotube Hybridization. Materials 2017, 10, 653. https://doi.org/10.3390/ma10060653
Liu X, Kang H, Wang Z, Zhang W, Li J, Zhang S. Simultaneously Toughening and Strengthening Soy Protein Isolate-Based Composites via Carboxymethylated Chitosan and Halloysite Nanotube Hybridization. Materials. 2017; 10(6):653. https://doi.org/10.3390/ma10060653
Chicago/Turabian StyleLiu, Xiaorong, Haijiao Kang, Zhong Wang, Wei Zhang, Jianzhang Li, and Shifeng Zhang. 2017. "Simultaneously Toughening and Strengthening Soy Protein Isolate-Based Composites via Carboxymethylated Chitosan and Halloysite Nanotube Hybridization" Materials 10, no. 6: 653. https://doi.org/10.3390/ma10060653
APA StyleLiu, X., Kang, H., Wang, Z., Zhang, W., Li, J., & Zhang, S. (2017). Simultaneously Toughening and Strengthening Soy Protein Isolate-Based Composites via Carboxymethylated Chitosan and Halloysite Nanotube Hybridization. Materials, 10(6), 653. https://doi.org/10.3390/ma10060653