Preparation and Enrichment Properties of Magnetic Dodecyl Chitosan/Silica Composite for Emerging Bisphenol Contaminants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
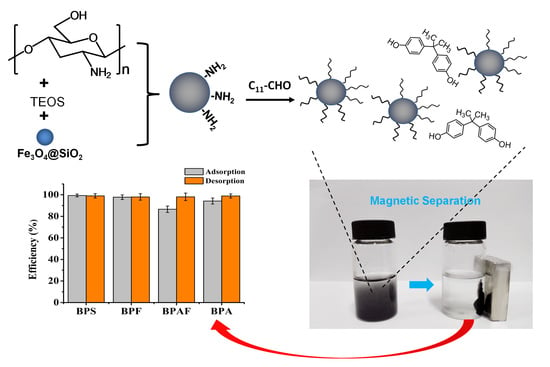
2.2. Preparation of Magnetic Dodecyl Chitosan/Silica Composite
2.3. Characterization Methods
2.4. Adsorption and Desorption Experiments
2.4.1. Batch Adsorption Experiments
2.4.2. Desorption and Regeneration Studies
2.5. HPLC Analysis
3. Results and Discussion
3.1. Characterization
3.2. The Influence of Long Alkyl Group on the Adsorption
3.3. Influence of pH on Adsorption
3.4. Adsorption Studies
3.4.1. Adsorption Kinetics
3.4.2. Adsorption Isotherm
3.5. Evaluation on the Enrichment Performance of the Resulting Composite
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Suvorov, A.; Waxman, D.J. Early programming of uterine tissue by bisphenol A: Critical evaluation of evidence from animal exposure studies. Reprod. Toxicol. 2015, 57, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, M.S.; Salim, M.R.; Lau, W.J.; Yusop, Z. A review on bisphenol A occurrences, health effects and treatment process via membrane technology for drinking water. Environ. Sci. Pollut. Res. 2016, 23, 1–19. [Google Scholar] [CrossRef] [PubMed]
- EFSA (European Food Safety Authority). Scientific opinion on the risks to public health related to the presence of bisphenol A (BPA) in foodstuffs. EFSA J. 2015, 13, 3978. [Google Scholar] [CrossRef]
- FDA (Food and Drug Administration). Indirect food additives: Adhesives and components of coatings. Federal Register 2013, 78, 41840–41843. [Google Scholar]
- Health Canada. Health Risk Assessment of Bisphenol a from Food Packaging Applications; Minister of Health: Ottawa, ON, Canada, 2008; pp. 1–13. [Google Scholar]
- Maćczak, A.; Cyrkler, M.; Bukowska, B.; Michałowicz, J. Bisphenol A, bisphenol S, bisphenol F and bisphenol AF induce different oxidative stress and damage in human red blood cells (in vitro study). Toxicol. In Vitro 2017, 41, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Mokra, K.; Kuźmińska-Surowaniec, A.; Woźniak, A.; Michałowicz, M. Evaluation of DNA-damaging potential of bisphenol A and its selected analogs in human peripheral blood mononuclear cells (in vitro study). Food Chem. Toxicol. 2017, 100, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Tišler, T.; Krel, A.; Gerželj, U.; Erjavec, B.; Dolenc, M.S.; Pintar, A. Hazard identification and risk characterization of bisphenols A, F and AF to aquatic organisms. Environ. Pollut. 2016, 212, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Liu, F.; Guo, Y.; Moon, H.B.; Nakata, H.; Wu, Q.; Kannan, K. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: Implications for human exposure. Environ. Sci. Technol. 2012, 46, 9138–9145. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lu, L.; Zhang, J.; Yang, Y.; Wu, Y.; Shao, B. Simultaneous determination of seven bisphenols in environmental water and solid samples by liquid chromatography-electrospray tandem mass spectrometry. J. Chromatogr. A 2014, 1328, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, J.; Wenzl, T. Development and validation of a stable-isotope dilution liquid chromatography-tandem mass spectrometry method for the determination of bisphenols in ready-made meals. J. Chromatogr. A 2015, 1414, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Gallo, P.; Pisciottano, I.D.M.; Esposito, F.; Fasano, E.; Scognamiglio, G.; Mita, G.D.; Cirillo, T. Determination of BPA, BPB, BPF, BADGE and BFDGE in canned energy drinks by molecularly imprinted polymer cleaning up and UPLC with fluorescence detection. Food Chem. 2017, 220, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Xian, Y.; Wu, Y.; Dong, H.; Guo, X.; Wang, B.; Wang, L. Dispersive micro solid phase extraction (DMSPE) using polymer anion exchange (PAX) as the sorbent followed by UPLC-MS/MS for the rapid determination of four bisphenols in commercial edible oils. J. Chromatogr. A 2017, 1517, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Gisi, S.D.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar]
- Zafar, R.; Zia, K.M.; Tabasum, S.; Jabeen, F.; Zuber, M. Polysaccharide based bionanocomposites, properties and applications: A review. Int. J. Biol. Macromol. 2016, 92, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; Caba, K.D.L. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Olivera, S.; Venkatesh, K.; Guna, V.K.; Gopalakrishna, K.; Yogesh, K.K. Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: A review. Carbohydr. Polym. 2016, 153, 600–618. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Manzoor, K.; Ikram, S. Versatile nature of hetero-chitosan based derivatives as biodegradable adsorbent for heavy metal ions: A review. Int. J Biol. Macromol. 2017, 105, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Dotto, G.L.; Rodrigues, F.K.; Tanabe, E.H.; Fröhlich, R.; Bertuol, D.A.; Martins, T.R.; Foletto, E.L. Development of chitosan/bentonite hybrid composite to remove hazardous anionic and cationic dyes from colored effluents. J. Environ. Chem. Eng. 2016, 4, 3230–3239. [Google Scholar] [CrossRef]
- Karaer, H.; Kaya, İ. Synthesis, characterization of magnetic chitosan/active charcoal composites and using at the adsorption of methylene blue and reactive blue4. Microporous Mesoporous Mater. 2016, 232, 26–38. [Google Scholar] [CrossRef]
- Feizbakhsh, A.; Ehteshami, S. Polythiophene-chitosan magnetic nanocomposite as a novel sorbent for disperse magnetic solid phase extraction of triazine herbicides in aquatic media. Chromatographia 2016, 79, 1177–1185. [Google Scholar] [CrossRef]
- Silva, S.R.D.; Albuquerque, N.J.A.D.; Almeida, R.M.D.; Abreu, F.C.D. Synthesis and charaterization of silica-based aldehyde chitosan hybrid material for biodiesel purification. Materials 2017, 10, 1132–1145. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Liang, J.; Cui, Y.; Xu, S.; Zhao, N. Fabrication of novel bioactive hydroxyapatite-chitosan-silica hybrid scaffolds: Combined the sol-gel method with 3D plotting technique. Carbohydr. Polym. 2018, 197, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Holzmeister, I.; Schamel, M.; Groll, J.; Gbureck, U.; Vorndran, E. Artificial inorganic biohybrids: The functional combination of microorganisms and cells with inorganic materials. Acta Biomater. 2018, 74, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, P.; Pietras, P.; Maciejewski, H. Synthesis, characterization, and thermal properties of organic-inorganic hybrids based on gelatin and organomodified silicones. Adv. Polym. Technol. 2015, 33, 36–38. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Yun, Y.S. Spinel ferrite magnetic adsorbents: Alternative future materials for water purification. Coord. Chem. Rev. 2016, 315, 90–111. [Google Scholar] [CrossRef]
- Fan, C.; Li, K.; He, Y.; Wang, Y.; Qian, X.; Jia, J. Evaluation of magnetic chitosan beads for adsorption of heavy metal ions. Sci. Total Environ. 2018, 627, 1396–1403. [Google Scholar] [CrossRef]
- Hamza, M.F.; Aly, M.M.; Abdelrahman, A.A.H.; Ramadan, S.; Raslan, H.; Wang, S.; Vincent, T.; Guibal, E. Functionalization of magnetic chitosan particles for the sorption of U(VI), Cu(II) and Zn(II)-hydrazide derivative of glycine-grafted chitosan. Materials 2017, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Ramin, S. Effective removal of copper from aqueous solutions by modified magnetic chitosan/graphene oxide nanocomposites. Int. J Biol. Macromol. 2018, 113, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Hao, K.; Han, F.; Tang, Z.; Niu, B.; Zhang, X.; Wang, Z.; Hong, S. Enhanced removal of bisphenol-AF onto chitosan-modified zeolite by sodium cholate in aqueous solutions. Carbohydr. Polym. 2015, 130, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.H.; Ghadermazi, M.; Bhatnagar, A.; Sadighara, P.; Jahed-Khaniki, G.; Heibati, B.; McKay, G. Adsorptive removal of endocrine disrupting bisphenol A from aqueous solution using chitosan. J. Environ. Chem. Eng. 2016, 4, 2647–2655. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Açıkyıldız, M.; Gürses, A.; Güneş, K.; Yalvaç, D. A comparative examination of the adsorption mechanism of an anionic textile dye (RBY 3GL) onto the powdered activated carbon (PAC) using various the isotherm models and kinetics equations with linear and non-linear methods. Appl. Surf. Sci. 2015, 354, 279–284. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed]









| C0 (mg/L) | Pseudo-First Order | Pseudo-Second Order | ||
|---|---|---|---|---|
| k1 (min−1) | R2 | k2 (g·mg−1·min−1) | R2 | |
| 10 | 0.0868 | 0.8439 | 0.6828 | 0.9986 |
| 20 | 0.2095 | 0.8796 | 0.4495 | 0.9998 |
| 40 | 0.2319 | 0.9149 | 0.3386 | 0.9999 |
| 50 | 0.1858 | 0.9630 | 0.1838 | 0.9999 |
| Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|
| qm | KL | R2 | 1/n | InKF | R2 |
| 2.188 | 0.8064 | 0.9792 | 0.8236 | 0.515 | 0.9947 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Liu, W.; Liu, H.; Wu, L.; Zhang, H. Preparation and Enrichment Properties of Magnetic Dodecyl Chitosan/Silica Composite for Emerging Bisphenol Contaminants. Materials 2018, 11, 1881. https://doi.org/10.3390/ma11101881
Hu J, Liu W, Liu H, Wu L, Zhang H. Preparation and Enrichment Properties of Magnetic Dodecyl Chitosan/Silica Composite for Emerging Bisphenol Contaminants. Materials. 2018; 11(10):1881. https://doi.org/10.3390/ma11101881
Chicago/Turabian StyleHu, Jingrong, Wangwei Liu, Huiling Liu, Lamei Wu, and Huijuan Zhang. 2018. "Preparation and Enrichment Properties of Magnetic Dodecyl Chitosan/Silica Composite for Emerging Bisphenol Contaminants" Materials 11, no. 10: 1881. https://doi.org/10.3390/ma11101881
APA StyleHu, J., Liu, W., Liu, H., Wu, L., & Zhang, H. (2018). Preparation and Enrichment Properties of Magnetic Dodecyl Chitosan/Silica Composite for Emerging Bisphenol Contaminants. Materials, 11(10), 1881. https://doi.org/10.3390/ma11101881