A Facile Flow-Casting Production of Bioactive Glass Coatings on Porous Titanium for Bone Tissue Engineering
Abstract
:1. Introduction
2. Experimental
2.1. Materials
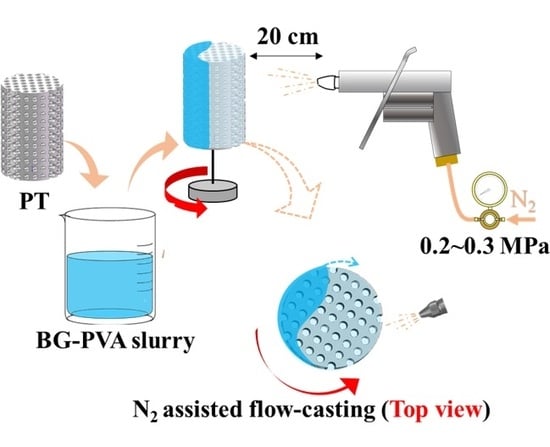
2.2. Fabrication of PT
2.3. Flow-Casting Process
2.4. Coating Characterizations
2.5. Cellular Studies
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lopez-Heredia, M.A.; Sohier, J.; Gaillard, C.; Quillard, S.; Dorget, M.; Layrolle, P. Rapid prototyped porous titanium coated with calcium phosphate as a scaffold for bone tissue engineering. Biomaterials 2008, 29, 2608–2615. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Xu, S.Q.; Zhou, S.W.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Schulze, C.; Weinmann, M.; Schweigel, C.; Kessler, O.; Bader, R. Mechanical properties of a newly additive manufactured implant material based on Ti-42Nb. Materials 2018, 11, 124. [Google Scholar] [CrossRef] [PubMed]
- Zadpoor, A.A. Bone tissue regeneration: The role of scaffold geometry. Biomater. Sci. 2015, 3, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Song, W.; Han, T.X.; Yan, J.; Li, F.P.; Zhao, L.Z.; Kou, H.C.; Zhang, Y.M. Influence of pore size of porous titanium fabricated by vacuum diffusion bonding of titanium meshes on cell penetration and bone ingrowth. Acta Biomater. 2016, 33, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Markhoff, J.; Wieding, J.; Weissmann, V.; Pasold, J.; Jonitz-Heincke, A.; Bader, R. Influence of different three-dimensional open porous pitanium scaffold designs on human osteoblasts behavior in static and dynamic cell investigations. Materials 2015, 8, 5490–5507. [Google Scholar] [CrossRef] [PubMed]
- Ince, A.; Rupp, J.; Frommelt, L.; Katzer, A.; Gille, J.; Lohr, J.F. Is “aseptic” loosening of the prosthetic cup after total hip replacement due to nonculturable bacterial pathogens in patients with low-grade infection? Clin. Infect. Dis. 2004, 39, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Salinas, A.J.; Esbrit, P.; Vallet-Regi, M. A tissue engineering approach based on the use of bioceramics for bone repair. Biomater. Sci. 2013, 1, 40–51. [Google Scholar] [CrossRef]
- Cheng, D.A.; Liu, D.P.; Tang, T.H.; Zhang, X.R.; Jia, X.L.; Cai, Q.; Yang, X.P. Effects of Ca/P molar ratios on regulating biological functions of hybridized carbon nanofibers containing bioactive glass nanoparticles. Biomed. Mater. 2017, 12, 025019. [Google Scholar] [CrossRef] [PubMed]
- Vahabzadeh, S.; Roy, M.; Bandyopadhyay, A.; Bose, S. Phase stability and biological property evaluation of plasma sprayed hydroxyapatite coatings for orthopedic and dental applications. Acta Biomater. 2015, 17, 47–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuart, B.W.; Titman, J.J.; Gimeno-Fabra, M.; Ahmed, I.; Grant, D.M. Insights into structural characterisation and thermal properties of compositionally equivalent vapour-condensed and melt-quenched glasses. Mater. Des. 2016, 111, 174–184. [Google Scholar] [CrossRef]
- Offermanns, V.; Andersen, O.Z.; Sillassen, M.; Almtoft, K.P.; Andersen, I.H.; Kloss, F.; Foss, M. A comparative in vivo study of strontium-functionalized and SLActive™ implant surfaces in early bone healing. Int. J. Nanomed. 2018, 13, 2189–2197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Lan, Z.; Bo, L.; Yong, H. Enhancement in sustained release of Antimicrobial peptide from dual-diameter-structured TiO2 nanotubes for long-lasting antibacterial activity and cytocompatibility. ACS Appl. Mater. Interfaces 2017, 9, 9449–9461. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, J.; Versace, D.L.; Abbad-Andallousi, S.; Pires, R.; Azevedo, C.; Cenedese, P.; Dubot, P. Antibacterial properties of nanostructured Cu-TiO2 surfaces for dental implants. Biomater. Sci. 2017, 5, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, H.J.; Chen, C.Z.; Zhao, Z.H. Review of the biocompatibility of micro-arc oxidation coated titanium alloys. Mater. Des. 2015, 85, 640–652. [Google Scholar] [CrossRef]
- Chen, Q.; Jing, J.; Qi, H.; Ahmed, I.; Yang, H.; Liu, X.; Lu, T.L.; Boccaccini, A.R. Electric field-assisted orientation of short shosphate glass fibers on stainless steel for biomedical applications. ACS Appl. Mater. Interfaces 2018, 10, 11529–11538. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yang, Y.Y.; de Larraya, U.P.; Garmendia, N.; Virtanen, S.; Boccaccini, A.R. Electrophoretic co-deposition of cellulose nanocrystals-45S5 bioactive glass nanocomposite coatings on stainless steel. Appl. Surf. Sci. 2016, 362, 323–328. [Google Scholar] [CrossRef]
- Lee, H.; Liao, J.D.; Sivashanmugan, K.; Liu, B.H.; Weng, S.L.; Juang, Y.D.; Yao, C.K. Dual properties of zirconia coated porous titanium for a stiffness enhanced bio-scaffold. Mater. Des. 2017, 132, 13–21. [Google Scholar] [CrossRef]
- Yavari, S.A.; Loozen, L.; Paganelli, F.L.; Bakhshandeh, S.; Lietaert, K.; Groot, J.A.; Fluit, A.C.; Boel, C.H.E.; Alblas, J.; Vogely, H.C.; et al. Antibacterial behavior of additively manufactured porous titanium with nanotubular surfaces releasing silver Ions. ACS Appl. Mater. Interfaces 2016, 8, 17080–17089. [Google Scholar] [CrossRef] [PubMed]
- Kollath, V.O.; Chen, Q.; Mullens, S.; Luyten, J.; Traina, K.; Boccaccini, A.R.; Cloots, R. Electrophoretic deposition of hydroxyapatite and hydroxyapatite-alginate on rapid prototyped 3D Ti6Al4V scaffolds. J. Mater. Sci. 2016, 51, 2338–2346. [Google Scholar] [CrossRef]
- Yao, Q.Q.; Jing, J.J.; Zeng, Q.Y.; Lu, T.L.; Liu, Y.; Zheng, X.; Chen, Q. Bilayered BMP2 eluting coatings on graphene foam by electrophoretic deposition: Electroresponsive BMP2 release and enhancement of osteogenic differentiation. ACS Appl. Mater. Interfaces 2017, 9, 39962–39970. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, W.; Li, X.K.; Zhang, X.; Wang, C.R.; Meng, X.F.; Pei, Y.F.; Fan, X.L.; Lan, P.H.; Wang, C.H.; et al. Improving osteointegration and osteogenesis of three-dimensional porous Ti6AI4V scaffolds by polydopamine-assisted biomimetic hydroxyapatite coating. ACS Appl. Mater. Interfaces 2015, 7, 5715–5724. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Lu, F.; Zhu, W.J.; Wang, D.; Zhu, X.J.; Tan, G.X.; Wang, X.L.; Zhang, Y.; Li, L.H.; Ning, C.Y. Bio-inspired citrate functionalized apatite coating on rapid prototyped titanium scaffold. Appl. Surf. Sci. 2014, 313, 947–953. [Google Scholar] [CrossRef]
- Braem, A.; Neirinck, B.; Schrooten, J.; Van der Biest, O.; Vleugels, J. Biofunctionalization of porous titanium coatings through sol-gel impregnation with a bioactive glass-ceramic. Mater. Sci. Eng. C 2012, 32, 2292–2298. [Google Scholar] [CrossRef]
- Chai, Y.C.; Truscello, S.; Van Bael, S.; Luyten, F.P.; Vleugels, J.; Schrooten, J. Perfusion electrodeposition of calcium phosphate on additive manufactured titanium scaffolds for bone engineering. Acta Biomater. 2011, 7, 2310–2319. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Ku, S.H.; Lee, H.; Park, C.B. Mussel-inspired polydopamine coating as a universal route to hydroxyapatite crystallization. Adv. Funct. Mater. 2010, 20, 2132–2139. [Google Scholar] [CrossRef]
- Li, W.; Nooeaid, P.; Roether, J.A.; Schubert, D.W.; Boccaccini, A.R. Preparation and characterization of vancomycin releasing PHBV coated 45S5 bioglass®-based glass–ceramic scaffolds for bone tissue engineering. J. Eur. Ceram. Soc. 2014, 34, 505–514. [Google Scholar] [CrossRef]
- Peitl, O.; LaTorre, G.P.; Hench, L.L. Effect of crystallization on apatite-layer formation of bioactive glass 45S5. J. Biomed. Mater. Res. 1996, 30, 509–514. [Google Scholar]
- Gomez-Vega, J.M.; Saiz, E.; Tomsia, A.P.; Oku, T.; Suganuma, K.; Marshall, G.W.; Marshall, S.J. Novel bioactive functionally graded coatings on Ti6Al4V. Adv. Mater. 2000, 12, 894–898. [Google Scholar] [CrossRef]
- Jones, J.R. Reprint of: Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2015, 23, S53–S82. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.; Thomas, B.; Leinenbach, C.; Eifler, D.; Minay, E.J.; Boccaccini, A.R. The electrophoretic deposition of Bioglass® particles on stainless steel and Nitinol substrates. Surf. Coat. Technol. 2006, 200, 4835–4845. [Google Scholar] [CrossRef]
- Chen, Q.; Garcia, R.P.; Munoz, J.; de Larraya, U.P.; Garmendia, N.; Yao, Q.Q.; Boccaccini, A.R. Cellulose nanocrystals-bioactive glass hybrid coating as bone substitutes by electrophoretic co-deposition: In situ control of mineralization of bioactive glass and enhancement of osteoblastic performance. ACS Appl. Mater. Interfaces 2015, 7, 24715–24725. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Cordero-Arias, L.; Roether, J.A.; Cabanas-Polo, S.; Virtanen, S.; Boccaccini, A.R. Alginate/Bioglass® composite coatings on stainless steel deposited by direct current and alternating current electrophoretic deposition. Surf. Coat. Technol. 2013, 233, 49–56. [Google Scholar] [CrossRef]
- Li, Q.Y.; He, G. Gelatin-enhanced porous titanium loaded with gentamicin sulphate and in vitro release behavior. Mater. Des. 2016, 99, 459–466. [Google Scholar] [CrossRef]
- Anglin, C.; Tolhurst, P.; Wyss, U.P.W.; Pichora, D.R. Glenoid cancellous bone strength and modulus. J. Biomech. 1999, 32, 1091–1097. [Google Scholar] [CrossRef]
- Katz, J.L. Anisotropy of Young’s modulus of bone. Nature 1980, 283, 106–107. [Google Scholar] [CrossRef] [PubMed]
- Cowin, S.C. Bone Mechanics Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Wang, Q.; Cheng, M.Q.; He, G.; Zhang, X.L. Surface modification of porous titanium with microarc oxidation and its effects on osteogenesis activity in vitro. J. Nanomater. 2015, 16, 30. [Google Scholar] [CrossRef]
- Li, X.; Ma, X.Y.; Feng, Y.F.; Wang, L.; Wang, C.T. A novel composite scaffold consisted of porous titanium and chitosan sponge for load-bearing applications: Fabrication, characterization and cellular activity. Compos. Sci. Technol. 2015, 117, 78–84. [Google Scholar] [CrossRef]
- Chen, Y.H.; Frith, J.E.; Dehghan-Manshadi, A.; Attar, H.; Kent, D.; Soro, N.D.M.; Bermingham, M.J.; Dargusch, M.S. Mechanical properties and biocompatibility of porous titanium scaffolds for bone tissue engineering. J. Mech. Behav. Biomed. 2017, 75, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, L.; Liu, R.; Lin, Y.; Chen, S.; Lu, S.; Lin, Z.; Chen, Z.; Wu, C.; Xiao, Y. The osteoimmunomodulatory property of a barrier collagen membrane and its manipulation via coating nanometer-sized bioactive glass to improve guided bone regeneration. Biomater. Sci. 2018, 6, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.N.; Lim, K.T.; Kim, Y.; Seonwoo, H.; Park, S.H.; Lim, H.J.; Kim, D.H.; Suh, K.Y.; Choung, P.H.; et al. Designing nanotopographical density of extracellular matrix for controlled morphology and function of human mesenchymal stem cells. Sci. Rep. 2013, 3, 3552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]






| Material | Processing Method | Porosity (vol %) | Elastic Modulus (GPa) | Ref. |
|---|---|---|---|---|
| Bare PT | Selective laser melting (SLM) | 80 ± 3 | 2.71 ± 0.14 | Our work |
| BG200-coated PT | SLM + annealed BG coating | 3.33 ± 0.20 | Our work | |
| Bare PT | Ti-wire entanglement | 44.7 | 1.05 | [38] |
| Bare PT | Electron beam melting (EBM) | 60 | 5.48 ± 0.54 | [39] |
| 67 | 3.85 ± 0.56 | |||
| 75 | 2.23 ± 0.68 | |||
| Bare PT | Powder metallurgy | 30 | 44.2 ± 0.6 | [40] |
| 40 | 24.7 ± 2.5 | |||
| 50 | 15.4 ± 1.1 | |||
| PT filled with gelatin gel | Ti-wire entanglement | 50 | 7.4 | [34] |
| 60 | 3.9 | |||
| 70 | 2.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Zhu, Q.; Qi, H.; Liu, X.; Ma, M.; Chen, Q. A Facile Flow-Casting Production of Bioactive Glass Coatings on Porous Titanium for Bone Tissue Engineering. Materials 2018, 11, 1540. https://doi.org/10.3390/ma11091540
Yang H, Zhu Q, Qi H, Liu X, Ma M, Chen Q. A Facile Flow-Casting Production of Bioactive Glass Coatings on Porous Titanium for Bone Tissue Engineering. Materials. 2018; 11(9):1540. https://doi.org/10.3390/ma11091540
Chicago/Turabian StyleYang, Haiou, Qijie Zhu, Hongfei Qi, Xianhu Liu, Meixia Ma, and Qiang Chen. 2018. "A Facile Flow-Casting Production of Bioactive Glass Coatings on Porous Titanium for Bone Tissue Engineering" Materials 11, no. 9: 1540. https://doi.org/10.3390/ma11091540
APA StyleYang, H., Zhu, Q., Qi, H., Liu, X., Ma, M., & Chen, Q. (2018). A Facile Flow-Casting Production of Bioactive Glass Coatings on Porous Titanium for Bone Tissue Engineering. Materials, 11(9), 1540. https://doi.org/10.3390/ma11091540