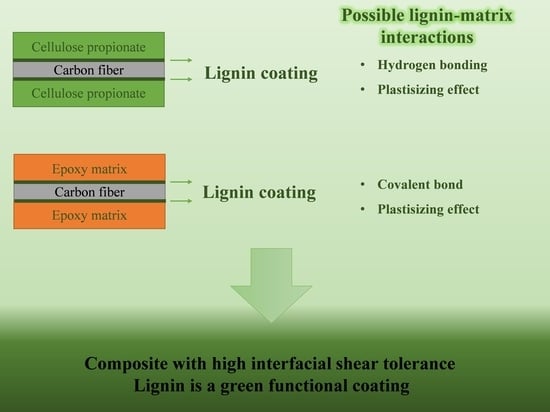
Lignin as a Functional Green Coating on Carbon Fiber Surface to Improve Interfacial Adhesion in Carbon Fiber Reinforced Polymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesizing Lignin Derivatives
2.2.2. Carbon Fiber Surface Modification
Grafting 4-(Aminomethyl)Benzene Functions onto the Carbon Fiber Surface
Derivatization of 4-(Aminomethyl)Benzene Functions for Determining the Grafting Density
Grafting Lignin Derivative onto the Carbon Fiber Surface
2.2.3. Mechanical Tests
Fragmentation Test
Microdroplet Test
2.2.4. Surface Analysis
3. Results and Discussion
3.1. Grafting Lignin onto the Carbon Fiber Surface
3.1.1. Quantifying Grafted 4-(Aminomethyl)Benzene Functions on the Surface
3.1.2. Surface Characterization—Experimental Evidence for Lignin Coating
3.2. Mechanical Tests
3.2.1. Fragmentation Test
3.2.2. Microdroplet Test
3.3. Practical Considerations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- U.S. Environmental Protection Agency. 2017 and later model year light-duty vehicle greenhouse gas emissions and corporate average fuel economy standards. Fed. Reg. 2012, 77, 62623–63200. [Google Scholar]
- Regulation of the European Parliament and of the Council. Setting Emission Performance Standards for New Passenger Cars and for New Light Commercial Vehicles as Part of the Union’s Integrated Approach to Reduce CO2 Emissions from Light-Duty Vehicles and Amending Regulation (EC) No 715/2007; European Parliament and of the Council: Brussels, Belgium, 2017. [Google Scholar]
- Kyono, T. Life cycle assessment of carbon fiber-reinforced plastic. In High-Performance and Specialty Fibers; The Society of Fiber Science and Technology, Japan, Ed.; Springer: Tokyo, Japan, 2016; pp. 355–361. ISBN 978-4-431-55202-4. [Google Scholar]
- Witten, E.; Sauer, M.; Kühnel, M. Composites Market Report 2017. Market Developments, Trends, Outlook and Challenges; Industrievereinigung Verstärkte Kunststoffe e.V. (Federation of Reinforced Plastics): Frankfurt, Germany, 2017. [Google Scholar]
- Fang, W.; Yang, S.; Wang, X.-L.; Yuan, T.-Q.; Sun, R.-C. Manufacture and application of lignin-based carbon fibers (LCFs) and lignin-based carbon nanofibers (LCNFs). Green Chem. 2017, 19, 1794–1827. [Google Scholar] [CrossRef]
- Yao, S.-S.; Jin, F.-L.; Rhee, K.Y.; Hui, D.; Park, S.-J. Recent advances in carbon-fiber-reinforced thermoplastic composites: A review. Compos. Part B Eng. 2018, 142, 241–250. [Google Scholar] [CrossRef]
- Szabó, L.; Imanishi, S.; Kawashima, N.; Hoshino, R.; Takada, K.; Hirose, D.; Tsukegi, T.; Ninomiya, K.; Takahashi, K. Carbon fiber reinforced cellulose-based polymers: Intensifying interfacial adhesion between the fibre and the matrix. RSC Adv. 2018, 8, 22729–22736. [Google Scholar] [CrossRef]
- Szabó, L.; Imanishi, S.; Kawashima, N.; Hoshino, R.; Hirose, D.; Tsukegi, T.; Ninomiya, K.; Takahashi, K. Interphase engineering of a cellulose-based carbon fiber reinforced composite by applying click chemistry. Chem. Open 2018, 7, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Gao, S.; Mäder, E.; Sharma, H.; Wei, L.Y.; Bijwe, J. Carbon fiber surfaces and composite interphases. Compos. Sci. Technol. 2014, 102, 35–50. [Google Scholar] [CrossRef]
- Krager-Kocsis, J.; Mahmood, H.; Pegoretti, A. Recent advances in fiber/matrix interphase engineering for polymer composites. Prog. Mater. Sci. 2015, 73, 1–43. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Meng, L.; Fan, L.; Wu, G.; Ma, L.; Zhao, M.; Huang, Y. Carboxyl functionalization of carbon fibers via aryl diazonium reaction in molten urea to enhance interfacial shear strength. Appl. Surf. Sci. 2016, 362, 341–347. [Google Scholar] [CrossRef]
- Li, N.; Wu, Z.; Huo, L.; Zong, L.; Guo, Y.; Wang, J.; Jian, W. One-step functionalization of carbon fiber using in situ generated aromatic diazonium salts to enhance adhesion with PPBES resins. RSC Adv. 2016, 6, 70704–70714. [Google Scholar] [CrossRef]
- Wang, C.; Chen, L.; Li, J.; Sun, S.; Ma, L.; Wu, G.; Zhao, F.; Jiang, B.; Huang, Y. Enhancing the interfacial strength of carbon fiber reinforced epoxy composites by green grafting of poly(oxypropylene) diamines. Compos. Part A Appl. Sci. Manuf. 2017, 99, 58–64. [Google Scholar] [CrossRef]
- Zho, M.; Meng, L.; Ma, L.; Wu, G.; Xie, F.; Ma, L.; Wang, W.; Jiang, B.; Huang, Y. Stepwise growth of melamine-based dendrimers onto carbon fibers and the effects on interfacial properties of epoxy composites. Compos. Sci. Technol. 2017, 138, 144–150. [Google Scholar] [CrossRef]
- Wu, G.; Ma, L.; Wang, Y.; Liu, L.; Huang, Y. Interfacial properties and thermo-oxidative stability of carbon fiber reinforced methylphenylsilicone resin composites modified with polyhedral oligomeric silsesquioxanes in the interphase. RSC Adv. 2016, 6, 5032–5039. [Google Scholar] [CrossRef]
- Ma, L.; Meng, L.; Wu, G.; Wang, Y.; Zhao, M.; Zhang, C.; Huang, Y. Effects of bonding types of carbon fibers with branched polyethyleneimine on the interfacial microstructure and mechanical properties of carbon fiber/epoxy resin composites. Compos. Sci. Technol. 2015, 117, 289–297. [Google Scholar] [CrossRef]
- Servinis, L.; Henderson, L.C.; Andrighetto, L.M.; Huson, M.G.; Gengenbach, T.R.; Fox, B.L. A novel approach to functionalise pristine unsized carbon fibre using in situ generated diazonium species to enhance interfacial shear strength. J. Mater. Chem. A 2015, 3, 3360–3371. [Google Scholar] [CrossRef] [Green Version]
- Beggs, K.M.; Servinis, L.; Gengenbach, T.R.; Huson, M.G.; Fox, B.L.; Henderson, L.C. A systematic study of carbon fiber surface grafting via in situ diazonium generation for improved interfacial shear strength in epoxy matrix composites. Compos. Sci. Technol. 2015, 118, 31–38. [Google Scholar] [CrossRef]
- Servinis, L.; Gengenbach, T.R.; Huson, M.G.; Henderson, L.C.; Fox, B.L. A novel approach to the functionalisation of pristine carbon fibre using azomethine 1,3-dipolar cycloaddition. Aust. J. Chem. 2015, 2, 335–344. [Google Scholar] [CrossRef]
- Servinis, L.; Beggs, K.M.; Scheffler, C.; Wölfel, E.; Randall, J.D.; Gegenbach, T.R.; Demir, B.; Walsh, T.R.; Doeven, E.H.; Francis, P.S. Electrochemical surface modification of carbon fibers by grafting of amine, carboxylic acid and lipophilic amide groups. Carbon 2017, 118, 393–403. [Google Scholar] [CrossRef]
- Servinis, L.; Beggs, K.M.; Gengenbach, T.R.; Doeven, E.H.; Francis, P.S.; Fox, B.L.; Pringle, J.M.; Pozo-Gonzalo, C.; Walsh, T.R.; Henderson, L.C. Tailoring the fiber-to-matrix interface using click chemistry on carbon fibre surfaces. J. Mater. Chem. A 2017, 5, 11204–11213. [Google Scholar] [CrossRef]
- Eykens, D.J.; Stojcevski, F.; Hendlmeier, A.; Arnold, C.L.; Randall, J.D.; Perus, M.D.; Servinis, L.; Gegenbach, T.R.; Demir, B.; Walsh, T.R. An efficient high-throughput grafting procedure for enhancing carbon fiber-to-matrix interactions in composites. Chem. Eng. J. 2018, 353, 373–380. [Google Scholar] [CrossRef]
- Arnold, C.L.; Beggs, K.M.; Eykens, D.J.; Stojcevski, F.; Servinis, L.; Henderson, L.C. Enhancing interfacial shear strength via surface grafting of carbon fibers using the Kolbe decarboxylation reaction. Compos. Sci. Technol. 2018, 159, 135–141. [Google Scholar] [CrossRef]
- Kümmerer, K. Sustainable chemistry: A future guiding principle. Angew. Chem. Int. Ed. 2017, 56, 16420–16421. [Google Scholar] [CrossRef] [PubMed]
- Raquez, J.; Deléglise, M.; Lacrampe, M.; Krawczak, P. Thermosetting (bio) materials derived from renewable resources: A critical review. Prog. Polym. Sci. 2010, 35, 487–509. [Google Scholar] [CrossRef]
- Dai, J.; Peng, Y.; Teng, N.; Liu, Y.; Liu, C.; Shen, X.; Mahmud, S.; Zhu, J.; Liu, X. High-performing and fire-resistant biobased epoxy resin from renewable sources. ACS Sustain. Chem. Eng. 2018, 6, 7589–7599. [Google Scholar] [CrossRef]
- Li, R.J.; Gutierrez, J.; Chung, Y.-L.; Frank, C.W.; Billington, S.L.; Sattely, E.S. A lignin-epoxy resin derived from biomass as an alternative to formaldehyde-based wood adhesives. Green Chem. 2018, 20, 1459–1466. [Google Scholar] [CrossRef]
- Nazhad, H.Y.; Thakur, V.K. Effect of morphological changes due to increasing carbon nanoparticles content on the quasi-static mechanical response of epoxy resin. Polymers 2018, 10, 1106. [Google Scholar] [CrossRef]
- Thakur, S.; Govender, P.P.; Mamo, M.A.; Tamulevicius, S.; Mishra, Y.K.; Thakur, V.K. Progress in lignin hydrogels and nanocomposites for water purification: Future perspectives. Vacuum 2017, 146, 342–355. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modifications of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Rials, T.G.; Glasser, W.G. Multiphase materials with lignin. VI. Effect of cellulose derivative structure on blend morphology with lignin. Wood Fiber Sci. 1989, 2, 80–90. [Google Scholar] [CrossRef]
- Eykens, D.J.; Servinis, L.; Scheffler, C.; Wölfel, E.; Demir, B.; Walsh, T.R.; Henderson, L.C. Synergistic interfacial effects of ionic liquids as sizing agents and surface modified carbon fibers. J. Mater. Chem. A 2018, 6, 4504–4514. [Google Scholar] [CrossRef]
- Granata, A.; Argyropoulos, D.S. 2-Chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane, a reagent for the accurate determination of the uncondensed and condensed phenolic moieties in lignins. J. Agric. Food Chem. 1995, 43, 1538–1544. [Google Scholar] [CrossRef]
- Kelly, A.; Tyson, A.W. Tensile properties of fiber-reinforced metals: Copper/tungsten and copper/molybdenum. J. Mech. Phys. Solids 1965, 13, 329–350. [Google Scholar] [CrossRef]
- Lopattananon, N.; Kettle, A.P.; Tripathi, D.; Beck, A.J.; Duval, E.; France, R.M.; Short, R.D.; Jones, F.R. Interface molecular engineering of carbon-fiber composites. Compos. Part A Appl. Sci. Manuf. 1999, 30, 49–57. [Google Scholar] [CrossRef]
- Naito, K. Fracture Behaviour of Continuous Carbon Fibre. In Improvement of Resin Impregnation Property and Reliability Evaluation of CFRP (Carbon Fiber Reinforced Plastic); Technical Information Institute Co., Ltd.: Tokyo, Japan, 2010; pp. 34–46. [Google Scholar]
- Galli, C. Radical reactions of arenediazonium ions: An easy entry into the chemistry of the aryl radical. Chem. Rev. 1988, 88, 765–792. [Google Scholar] [CrossRef]
- Bahr, J.L.; Tour, J.M. Highly functionalized carbon nanotubes using in situ generated diazonium compounds. Chem. Mater. 2001, 13, 3823–3824. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Meng, L.; Fan, D.; He, J.; Yu, J.; Qi, M.; Chen, Z.; Huang, Y. Interfacial enhancement of carbon fiber composites by generation 1-3 dendritic hexamethylenetetramine functionalization. Appl. Surf. Sci. 2014, 296, 61–68. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, S.; Yang, D.; Dodelet, J.-P.; Sacher, E. The surface analytical characterization of carbon fibers functionalized by H2SO4/HNO3 treatment. Carbon 2008, 46, 196–205. [Google Scholar] [CrossRef]
- Ehlert, G.J.; Lin, Y.; Sodano, H.A. Carboxyl functionalization of carbon fibers through a grafting reaction that preserves fiber tensile strength. Carbon 2011, 49, 4246–4255. [Google Scholar] [CrossRef]
- Meier, A.R.; Bahureksa, W.A.; Heien, M.L. Elucidating the structure-function relationship of poly(3,4-theylenedioxythiophene) films to advance electrochemical measurements. J. Phys. Chem. C 2016, 120, 2114–21122. [Google Scholar] [CrossRef]
- Marsella, J.A.; Starner, W.E. Acceleration of amine/epoxy reactions with N-methyl secondary amines. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 921–930. [Google Scholar] [CrossRef]
- Wu, Q.; Li, M.; Gu, Y.; Wang, S.; Yao, L.; Zhang, Z. Effect of sizing agent on interfacial adhesion of commercial high strength carbon fiber-reinforced resin composites. Polym. Compos. 2016, 37, 254–261. [Google Scholar] [CrossRef]
- Huson, M.G.; Church, J.S.; Kafi, A.A.; Woodhead, A.L.; Khoo, J.; Kiran, M.S.R.N.; Bradby, J.E.; Fox, B.L. Heterogeneity of carbon fibre. Carbon 2014, 68, 240–249. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, L.; Imanishi, S.; Tetsuo, F.; Hirose, D.; Ueda, H.; Tsukegi, T.; Ninomiya, K.; Takahashi, K. Lignin as a Functional Green Coating on Carbon Fiber Surface to Improve Interfacial Adhesion in Carbon Fiber Reinforced Polymers. Materials 2019, 12, 159. https://doi.org/10.3390/ma12010159
Szabó L, Imanishi S, Tetsuo F, Hirose D, Ueda H, Tsukegi T, Ninomiya K, Takahashi K. Lignin as a Functional Green Coating on Carbon Fiber Surface to Improve Interfacial Adhesion in Carbon Fiber Reinforced Polymers. Materials. 2019; 12(1):159. https://doi.org/10.3390/ma12010159
Chicago/Turabian StyleSzabó, László, Sari Imanishi, Fujie Tetsuo, Daisuke Hirose, Hisai Ueda, Takayuki Tsukegi, Kazuaki Ninomiya, and Kenji Takahashi. 2019. "Lignin as a Functional Green Coating on Carbon Fiber Surface to Improve Interfacial Adhesion in Carbon Fiber Reinforced Polymers" Materials 12, no. 1: 159. https://doi.org/10.3390/ma12010159
APA StyleSzabó, L., Imanishi, S., Tetsuo, F., Hirose, D., Ueda, H., Tsukegi, T., Ninomiya, K., & Takahashi, K. (2019). Lignin as a Functional Green Coating on Carbon Fiber Surface to Improve Interfacial Adhesion in Carbon Fiber Reinforced Polymers. Materials, 12(1), 159. https://doi.org/10.3390/ma12010159