Green Biosynthesis of Silver Nanoparticles Using Eriobotrya japonica (Thunb.) Leaf Extract for Reductive Catalysis
Abstract
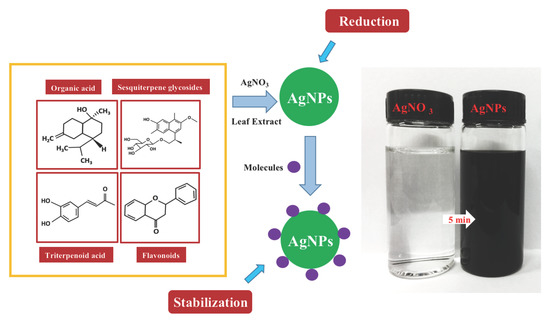
:1. Introduction
2. Materials and Methods
2.1. Preparation of Leaf Extract and Chemicals
2.2. Green Biosynthesis of Ag Nanoparticles
2.3. Characterization Method and Instrument
2.4. Catalytic Activity of AgNPs
3. Results and Discussion
3.1. Confirmation of AgNPs Formation using UV-Visible Spectroscopy Analysis
3.2. TEM, FESEM, and SAED Analyses
3.3. XRD, FTIR, and EDS Analysis of Biogenic AgNPs
3.4. Reductive Degradation of Reactive Dyes by Biogenic AgNPs
3.4.1. Compare of Degradation Ability of Different Catalysts
3.4.2. Evaluation of Reductive Degradation Activity of Reactive Dyes
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Song, J.Y.; Kim, B.S. Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioproc. Biosyst. Eng. 2009, 32, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Izadiyan, Z.; Shameli, K.; Hara, H.; Taib, S.H.M. Cytotoxicity assay of biosynthesis gold nanoparticles mediated by walnut (Juglans regia) green husk extract. J. Mol. Struct. 2018, 1151, 97–105. [Google Scholar] [CrossRef]
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bharagava, R.N.; Banu, J.R.; Shin, H.S. A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids Surf. B Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Mirtaheri, B.; Shokouhimehr, M.; Beitollahi, A. Synthesis of mesoporous tungsten oxide by template-assisted sol-gel method and its photocatalytic degradation activity. J. Sol. Gel. Sci. Technol. 2017, 82, 148–156. [Google Scholar] [CrossRef]
- Haghighatzadeh, A.; Mazinani, B.; Shokouhimehr, M.; Samiee, L. Preparation mesoporous TiO2-SiO2 by ultrasonic impregnation method and effect of its calcination temperature on photocatalytic activity. Desalin. Water Treat. 2017, 92, 145–151. [Google Scholar] [CrossRef]
- Gopinath, V.; MubarakAli, D.; Priyadarshini, S.; Priyadharsshini, N.M.; Thajuddin, N.; Velusamy, P. Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: A novel biological approach. Colloid Surf. B 2012, 96, 69–74. [Google Scholar] [CrossRef]
- Aragay, G.; Merkoci, A. Nanomaterials application in electrochemical detection of heavy metals. Electrochim. Acta 2012, 84, 49–61. [Google Scholar] [CrossRef]
- Xu, S.; Chen, S.; Zhang, F.; Jiao, C.; Song, J.; Chen, Y.; Lin, H.; Gotoh, Y.; Morikawa, H. Preparation and controlled coating of hydroxyl-modified silver nanoparticles on silk fibers through intermolecular interaction-induced self-assembly. Mater. Des. 2016, 95, 107–118. [Google Scholar] [CrossRef]
- Rajan, A.; Vilas, V.; Philip, D. Catalytic and antioxidant properties of biogenic silver nanoparticles synthesized using Areca catechu nut. J. Mol. Liq. 2015, 207, 231–236. [Google Scholar] [CrossRef]
- Rasheed, T.; Bilal, M.; Iqbal, H.M.N.; Li, C.L. Green biosynthesis of silver nanoparticles using leaves extract of Artemisia vulgaris and their potential biomedical applications. Colloid Surf. B 2017, 158, 408–415. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interfaces 2010, 156, 1–13. [Google Scholar] [CrossRef]
- Roya, S.; Das, T.K.; Maiti, G.P.; Basu, U. Microbial biosynthesis of nontoxic gold nanoparticles. Mater. Sci. Eng. B Adv. 2016, 203, 41–51. [Google Scholar] [CrossRef]
- Pradeep, T.; Anshup. Noble metal nanoparticles for water purification: A critical review. Thin Solid Films 2009, 517, 6441–6478. [Google Scholar] [CrossRef]
- Pandey, S.; Mewada, A.; Thakur, M.; Shah, R.; Oza, G.; Sharon, M. Biogenic gold nanoparticles as fotillas to fire berberine hydrochloride using folic acid as molecular road map. Mat. Sci. Eng. C Mater. 2013, 33, 3716–3722. [Google Scholar] [CrossRef] [PubMed]
- Rostami, H.; Khosravi, F.; Mohseni, M.; Rostami, A.A. Biosynthesis of Ag nanoparticles using isolated bacteria from contaminated sites and its application as an efficient catalyst for hydrazine electrooxidation. Int. J. Biol. Macromol. 2018, 107, 343–348. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Alharbi, R.M.; Al-Enazi, N.M.; Alkhulaifi, M.M.; Ibraheem, I.B.M. Rapid biosynthesis of silver nanoparticles using the marine red alga Laurencia catarinensis and their characterization. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 150–157. [Google Scholar] [CrossRef]
- Arunachalam, R.; Dhanasingh, S.; Kalimuthu, B.; Uthirappan, M.; Rose, C.; Mandal, A.B. Phytosynthesis of silver nanoparticles using Coccinia grandis leaf extract and its application in the photocatalytic degradation. Colloids Surf. B Biointerfaces 2012, 94, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Ping, Y.; Zhang, J.; Xing, T.; Chen, G.; Tao, R.; Choo, K.-H. Green synthesis of silver nanoparticles using grape seed extract and their application for reductive catalysis of Direct Orange 26. J. Ind. Eng. Chem. 2018, 58, 74–79. [Google Scholar] [CrossRef]
- Sangaonkar, G.M.; Pawar, K.D. Garcinia indica mediated biogenic synthesis of silver nanoparticles with antibacterial and antioxidant activities. Colloid Surf. B 2018, 164, 210–217. [Google Scholar] [CrossRef]
- Jung, H.A.; Park, J.C.; Chung, H.Y.; Kim, J.; Choi, J.S. Antioxidant flavonoids and chlorogenic acid from the leaves of Eriobotrya japonica. Arch. Pharmacal Res. 1999, 22, 213. [Google Scholar] [CrossRef]
- Chen, J.; Li, W.L.; Wu, J.L.; Ren, B.R.; Zhang, H.Q. Euscaphic acid, a new hypoglycemic natural product from Folium Eriobotryae. Pharmazie 2008, 63, 765–767. [Google Scholar] [PubMed]
- Zhao, L.; Chen, J.; Lv, H.; Ao, X.C.; Ren, B.R.; Li, W.L. A New Sesquiterpene Glycoside from the Leaves of Eriobotrya japonica. Chem. Nat. Compd. 2015, 51, 1103–1106. [Google Scholar] [CrossRef]
- Guilger, M.; Pasquoto-Stigliani, T.; Bilesky-Jose, N.; Grillo, R.; Abhilash, P.C.; Fraceto, L.F.; de Lima, R. Biogenic silver nanoparticles based on trichoderma harzianum: Synthesis, characterization, toxicity evaluation and biological activity. Sci. Rep. 2017, 7, 44421. [Google Scholar] [CrossRef] [PubMed]
- Panacek, A.; Kvitek, L.; Prucek, R.; Kolar, M.; Vecerova, R.; Pizurova, N.; Sharma, V.K.; Nevecna, T.; Zboril, R. Silver colloid nanoparticles: Synthesis, characterization, and their antibacterial activity. J. Phys. Chem. B 2006, 110, 16248–16253. [Google Scholar] [CrossRef]
- Yue, X.X.; Lin, H.T.; Yan, T.; Zhang, D.S.; Lin, H.; Chen, Y.Y. Synthesis of Silver Nanoparticles with Sericin and Functional Finishing to Cotton Fabrics. Fibers Polym. 2014, 15, 716–722. [Google Scholar] [CrossRef]
- Bogireddy, N.K.R.; Kiran Kumar, H.A.; Mandal, B.K. Biofabricated silver nanoparticles as green catalyst in the degradation of different textile dyes. J. Environ. Chem. Eng. 2016, 4, 56–64. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Ichwan, S.J.A.; Parine, N.R.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Intracellular biosynthesis of Au and Ag nanoparticles using ethanolic extract of Brassica oleracea L. and studies on their physicochemical and biological properties. J. Environ. Sci. 2015, 29, 151–157. [Google Scholar] [CrossRef]
- Jensen, T.R.; Malinsky, M.D.; Haynes, C.L.; Van Duyne, R.P. Nanosphere lithography: Tunable localized surface plasmon resonance spectra of silver nanoparticles. J. Phys. Chem. B 2000, 104, 10549–10556. [Google Scholar] [CrossRef]
- Manjari Mishra, P.; Kumar Sahoo, S.; Kumar Naik, G.; Parida, K. Biomimetic synthesis, characterization and mechanism of formation of stable silver nanoparticles using Averrhoa carambola L. leaf extract. Mater. Lett. 2015, 160, 566–571. [Google Scholar] [CrossRef]
- Kalimuthu, K.; Suresh Babu, R.; Venkataraman, D.; Bilal, M.; Gurunathan, S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf. B Biointerfaces 2008, 65, 150–153. [Google Scholar] [CrossRef]
- Mittal, A.K.; Bhaumik, J.; Kumar, S.; Banerjee, U.C. Biosynthesis of silver nanoparticles: Elucidation of prospective mechanism and therapeutic potential. J. Colloid Interface Sci. 2014, 415, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Hamano, F.; Seki, H.; Ke, M.; Gopiraman, M.; Lim, C.T.; Kim, I.S. Cellulose acetate nanofiber mat with honeycomb-like surface structure. Mater. Lett. 2016, 169, 33–36. [Google Scholar] [CrossRef]
- Gopiraman, M.; Bang, H.; Yuan, G.; Yin, C.; Song, K.-H.; Lee, J.S.; Chung, I.M.; Karvembu, R.; Kim, I.S. Noble metal/functionalized cellulose nanofiber composites for catalytic applications. Carbohydr. Polym. 2015, 132, 554–564. [Google Scholar] [CrossRef]
- Kale, R.D.; Jagtap, P. Biogenic Synthesis of Silver Nanoparticles Using Citrus Limon Leaves and Its Structural Investigation; Springer: Singapore, 2018; pp. 11–20. [Google Scholar]
- Ramimoghadam, D.; Bagheri, S.; Hamid, S.B.A. Progress in electrochemical synthesis of magnetic iron oxide nanoparticles. J. Magn. Magn. Mater. 2014, 368, 207–229. [Google Scholar] [CrossRef]
- Magudapathy, P.; Gangopadhyay, P.; Panigrahi, B.K.; Nair, K.G.M.; Dhara, S. Electrical transport studies of Ag nanoclusters embedded in glass matrix. Phys. B 2001, 299, 142–146. [Google Scholar] [CrossRef]






| Colloid | Temperature | Proportion Ratio of Silver Nitrate and Leaf Extract (v:v) | pH |
|---|---|---|---|
| (°C) | (mL) | ||
| G-L1 | 20 | 10:1 | 7.0 |
| G-L2 | 20 | 2:1 | 7.5 |
| G-L3 | 20 | 1:1 | 8.0 |
| G-M1 | 50 | 10:1 | 7.5 |
| G-M2 | 50 | 2:1 | 8.0 |
| G-M3 | 50 | 1:1 | 7.0 |
| G-H1 | 80 | 10:1 | 8.0 |
| G-H2 | 80 | 2:1 | 7.0 |
| G-H3 | 80 | 1:1 | 7.5 |
| Influencing Factor | DEVSQ | DOF | F Value | F Critical-Value | Significance |
|---|---|---|---|---|---|
| Temperature (°C) | 91.436 | 2 | 63.016 | 5.140 | * |
| Proportion ratio of silver nitrate and leaf extract (v:v) | 1.622 | 2 | 1.000 | 5.140 | |
| pH | 0.228 | 2 | 1.118 | 5.140 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Tang, J.; Liu, X.; Ren, X.; Zhen, M.; Wang, L. Green Biosynthesis of Silver Nanoparticles Using Eriobotrya japonica (Thunb.) Leaf Extract for Reductive Catalysis. Materials 2019, 12, 189. https://doi.org/10.3390/ma12010189
Yu C, Tang J, Liu X, Ren X, Zhen M, Wang L. Green Biosynthesis of Silver Nanoparticles Using Eriobotrya japonica (Thunb.) Leaf Extract for Reductive Catalysis. Materials. 2019; 12(1):189. https://doi.org/10.3390/ma12010189
Chicago/Turabian StyleYu, Chen, Jingchun Tang, Xiaomei Liu, Xinwei Ren, Meinan Zhen, and Lan Wang. 2019. "Green Biosynthesis of Silver Nanoparticles Using Eriobotrya japonica (Thunb.) Leaf Extract for Reductive Catalysis" Materials 12, no. 1: 189. https://doi.org/10.3390/ma12010189
APA StyleYu, C., Tang, J., Liu, X., Ren, X., Zhen, M., & Wang, L. (2019). Green Biosynthesis of Silver Nanoparticles Using Eriobotrya japonica (Thunb.) Leaf Extract for Reductive Catalysis. Materials, 12(1), 189. https://doi.org/10.3390/ma12010189