Endocytosis and Lack of Cytotoxicity of Alkyl-Capped Silicon Quantum Dots Prepared from Porous Silicon
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cytotoxicity
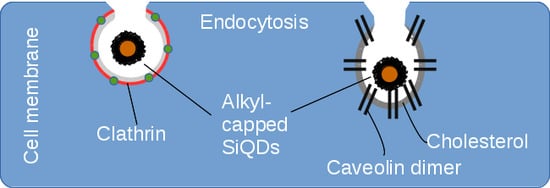
2.2. Endocytosis of Alkyl SiQDs
2.3. Caveolin Gene Expression
2.4. Chlorpromazine Inhibition
3. Materials and Methods
3.1. Preparation of Alkyl-SiQDs and Dispersions in Aqueous Media
3.2. CACO-2 Cell Culture
3.3. Optical and Epifluorescence Microscopy
3.4. Observation of Internalization of SiQDs by Epifluorescence Microscopy
3.5. CACO-2 Cell Viability (MTT Assay)
3.6. CACO-2 Intracellular ATP Content (FLASC Assay)
3.7. CACO-2 Oxidative Stress (H2DCFDA Assay)
3.8. CACO-2 DNA Damage (Comet Assay)
3.9. CACO-2 Chronic Exposure to Alkyl SiQDs
3.10. Internalization of SiQDs in Different Cell Lines
3.11. Specific Gene Expression of Caveolin 1 and Caveolin 2
3.11.1. RNA Preparation
3.11.2. cDNA Synthesis
3.11.3. Quantitative Reverse Transcriptase Real Time PCR Analysis
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Smith, A.M.; Gao, X.H.; Nie, S.M. Quantum dot nanocrystals for in vivo molecular and cellular imaging. Photochem. Photobiol. 2004, 80, 377–385. [Google Scholar] [CrossRef]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum Dots for Live Cells, in Vivo Imaging, and Diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, W.C.; Maxwell, D.J.; Gao, X.; Bailey, R.E.; Han, M.; Nie, S. Luminescent quantum dots for multiplexed biological detection and imaging. Curr. Opin. Biotechnol. 2002, 13, 40–46. [Google Scholar] [CrossRef]
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef]
- Kirchner, C.; Liedl, T.; Kudera, S.; Pellegrino, T.; Muñoz Javier, A.; Gaub, H.E.; Stölzle, S.; Fertig, N.; Parak, W.J. Cytotoxicity of colloidal CdSe and CdSe/ZnS nanoparticles. Nano Lett. 2005, 5, 331–338. [Google Scholar] [CrossRef]
- O’Farrell, N.; Houlton, A.; Horrocks, B.R. Silicon nanoparticles: Applications in cell biology and medicine. Int. J. Nanomed. 2006, 1, 451–472. [Google Scholar] [CrossRef]
- Das, A.; Snee, P.T. Synthetic Developments of Nontoxic Quantum Dots. ChemPhysChem 2016, 17, 598–617. [Google Scholar] [CrossRef]
- Park, J.-H.; Gu, L.; von Maltzahn, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat. Mat. 2009, 8, 331–336. [Google Scholar] [CrossRef]
- Coffer, J.L.; Whitehead, M.A.; Nagesha, D.K.; Mukherjee, P.; Akkaraju, G.; Totolici, M.; Saffie, R.S.; Canham, L.T. Porous silicon-based scaffolds for tissue engineering and other biomedical applications. Phys. Status Solidi A 2005, 202, 1451–1455. [Google Scholar] [CrossRef]
- Buriak, J.M. Organometallic chemistry on silicon and germanium surfaces. Chem. Rev. 2002, 102, 1271–1308. [Google Scholar] [CrossRef]
- Yu, Y.; Fan, G.; Fermi, A.; Mazzaro, R.; Morandi, V.; Smilgies, D.-M.; Korgel, B.A. Size-Dependent Photoluminescence Efficiency of Silicon Nanocrystal Quantum Dots. J. Phys. Chem. C 2017, 121, 23240–23248. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Swihart, M.T. Surface Functionalization of Silicon Nanoparticles Produced by Laser-Driven Pyrolysis of Silane followed by HF-HNO3 Etching. Langmuir 2004, 20, 4720–4727. [Google Scholar] [CrossRef]
- Clark, R.J.; Aghajamali, M.; Gonzalez, C.M.; Hadidi, L.; Islam, M.A.; Javadi, M.; Hosnay Mobarak, M.; Purkait, T.K.; Robidillo, C.J.T.; Sinelnikov, R.; et al. From Hydrogen Silsesquioxane to Functionalized Silicon Nanocrystals. Chem. Mater. 2017, 29, 80–89. [Google Scholar] [CrossRef]
- Lie, L.H.; Duerdin, M.; Tuite, E.M.; Houlton, A.; Horrocks, B.R. Preparation and characterisation of luminescent alkylated-silicon quantum dots. J. Electroanal. Chem. 2002, 538/539, 183–190. [Google Scholar] [CrossRef]
- Chao, Y.; Siller, L.; Krishnamurthy, S.; Coxon, P.R.; Bangert, U.; Gass, M.; Kjeldgaard, L.; Patole, S.N.; Lie, L.H.; O’Farrell, N.; et al. Evaporation and deposition of alkyl-capped silicon nanocrystals in ultrahigh vacuum. Nat. Nanotechnol. 2007, 2, 486–489. [Google Scholar] [CrossRef]
- Dickinson, F.M.; Alsop, T.A.; Al-Sharif, N.; Berger, C.E.M.; Datta, H.K.; Siller, L.; Chao, Y.; Tuite, E.M.; Houlton, A.; Horrocks, B.R. Dispersions of alkyl-capped silicon nanocrystals in aqueous media: Photoluminescence and ageing. Analyst 2008, 133, 1573–1580. [Google Scholar] [CrossRef]
- Zidek, K.; Pelant, I.; Trojanek, F.; Maly, P.; Gilliot, P.; Honerlage, B.; Oberle, J.; Siller, L.; Little, R.; Horrocks, B.R. Ultrafast stimulated emission due to quasidirect transitions in silicon nanocrystals. Phys. Rev. B 2011, 84, 085321. [Google Scholar] [CrossRef]
- Rostron, R.J.; Horrocks, B.R.; Roberts, G. Distributed luminescence from alkyl-capped silicon quantum dots. J. Appl. Phys. 2009, 105, 094302. [Google Scholar] [CrossRef]
- Siller, L.; Krishnamurthy, S.; Kjeldgaard, L.; Horrocks, B.R.; Chao, Y.; Houlton, A.; Chakraborty, A.K.; Hunt, M.R.C. Core and valence exciton formation in X-ray absorption, X-ray emission and X-ray excited optical luminescence from passivated Si nanocrystals at the Si L2,3 edge. J. Phys. Condens. Matter 2009, 21, 095005. [Google Scholar] [CrossRef]
- Al-Sharif, N.H.; Berger, C.E.M.; Varanasi, S.S.; Chao, Y.; Horrocks, B.R.; Datta, H.K. Alkyl–Capped Silicon Nanocrystals Lack Cytotoxicity and have Enhanced Intracellular Accumulation in Malignant Cells via Cholesterol-Dependent Endocytosis. Small 2009, 5, 221–228. [Google Scholar] [CrossRef]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; de Haan, L.H.J.; Evers, N.M.; Jiang, X.; Marcelis, A.T.M.; Zuilhof, H.; Rietjens, I.M.C.M.; Alink, G.M. Role of surface charge and oxidative stress in cytotoxicity of organic monolayer-coated silicon nanoparticles towards macrophage NR8383 cells. Part. Fibre Toxicol. 2010, 7, 1–12. [Google Scholar] [CrossRef]
- Shiohara, A.; Hanada, S.; Prabakar, S.; Fujioka, K.; Lim, T.H.; Yamamoto, K.; Northcote, P.T.; Tilley, R.D. Chemical reactions on surface molecules attached to silicon quantum dots. J. Am. Chem. Soc. 2010, 132, 248–253. [Google Scholar] [CrossRef]
- Wang, Q.; Bao, Y.; Zhang, X.; Coxon, P.R.; Jayasooriya, U.A.; Chao, Y. Uptake and Toxicity Studies of Poly-Acrylic Acid Functionalized Silicon Nanoparticles in Cultured Mammalian Cells. Adv. Healthc. Mater. 2012, 1, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Zhi, B.; Mishra, S.; Hudson-Smith, N.V.; Kortshagen, U.R.; Haynes, C.L. Toxicity Evaluation of Boron- and Phosphorus-Doped Silicon Nanocrystals toward Shewanella oneidensis MR-1. ACS Appl. Nano Mater. 2018, 1, 4884–4893. [Google Scholar] [CrossRef]
- Singh, N.; Manshian, B.; Jenkins, G.J.S.; Griffiths, S.M.; Williams, P.M.; Maffeis, T.G.G.; Wright, C.J.; Doak, S.H. NanoGenotoxicology: The DNA damaging potential of engineered nanomaterials. Biomaterials 2009, 30, 3891–3914. [Google Scholar] [CrossRef]
- Choi, J.; Zhang, Q.; Reipa, V.; Wang, N.S.; Stratmeyer, M.E.; Hitchins, V.M.; Goering, P.L. Comparison of cytotoxic and inflammatory responses of photoluminescent silicon nanoparticles with silicon micron-sized particles in RAW 264.7 macrophages. J. Appl. Toxicol. 2009, 29, 52–60. [Google Scholar] [CrossRef]
- Fischer, H.C.; Chan, W.C.W. Nanotoxicity: The growing need for in vivo study. Curr. Opin. Biotechnol. 2007, 18, 565–571. [Google Scholar] [CrossRef]
- Santos, H.A.; Bimbo, L.M.; Herranz, B.; Shahbazi, M.-A.; Hirvonen, J.; Salonen, J. Nanostructured porous silicon in preclinical imaging: Moving from bench to bedside. J. Mater. Res. 2013, 28, 152–164. [Google Scholar] [CrossRef]
- Doherty, G.J.; McMahon, H.T. Mechanisms of Endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef] [Green Version]
- Pinto, M.; Robine-Leon, S.; Appay, M.D.; Kedinger, M.; Triadou, N.; Dussaulx, E.; Lacroix, B.; Simon-Assmann, P.; Haffen, K.; Fogh, J.; et al. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol. Cell 1983, 47, 323–330. [Google Scholar]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef]
- Ahire, J.H.; Wang, Q.; Coxon, P.R.; Malhotra, G.; Brydson, R.; Chen, R.; Chao, Y. Highly Luminescent and Nontoxic Amine-Capped Nanoparticles from Porous Silicon: Synthesis and Their Use in Biomedical Imaging. ACS Appl. Mater. Interfaces 2012, 4, 3285–3292. [Google Scholar] [CrossRef]
- Gongalsky, M.B.; Osminkina, L.A.; Pereira, A.; Manankov, A.A.; Fedorenko, A.A.; Vasiliev, A.N.; Solovyen, V.V.; Kudryavtsev, A.A.; Sentis, M.; Kabashin, A.V.; et al. Laser-synthesized oxide-passivated bright Si quantum dots for bioimaging. Sci. Rep. 2016, 6, 24372. [Google Scholar] [CrossRef]
- Singh, S.; Bhatta, U.M.; Satyam, P.V.; Dhawan, A.; Sastry, M.; Prasad, B.L.V. Bacterial synthesis of silicon/silica nanocomposites. J. Mater. Chem. 2008, 18, 2601–2606. [Google Scholar] [CrossRef]
- Geng, X.; Li, Z.; Hu, Y.; Liu, H.; Sun, Y.; Meng, H.; Wang, Y.; Qu, L.; Lin, Y. One-Pot Green Synthesis of Ultrabright N-Doped Fluorescent Silicon Nanoparticles for Cellular Imaging by Using Ethylenediaminetetraacetic Acid Disodium Salt as an Effective Reductant. ACS Appl. Mater. Interfaces 2018, 10, 27979–27986. [Google Scholar] [CrossRef]
- Jensen, S.E.; Hubrechts, P.; Klein, B.M.; Haslov, K.R. Development and validation of an ATP method for rapid estimation of viable units in lyophilised BCG Danish 1331 vaccine. Biologicals 2008, 36, 308–314. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Liong, M.; Maedler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the Mechanism of Toxicity of Zinc Oxide and Cerium Oxide Nanoparticles Based on Dissolution and Oxidative Stress Properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef] [Green Version]
- Sayes, C.M.; Reed, K.L.; Warheit, D.B. Assessing toxicity of fine and nanoparticles: Comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol. Sci. 2007, 97, 163–180. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef]
- Nel, A.E.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Scott, B.L.; Sochacki, K.A.; Low-Nam, S.T.; Bailey, E.M.; Luu, Q.; Hor, A.; Dickey, A.M.; Smith, S.; Kerkvliet, J.G.; Taraska, J.W.; et al. Membrane bending occurs at all stages of clathrin-coat assembly and defines endocytic dynamics. Nat. Commun. 2018, 9, 419. [Google Scholar] [CrossRef]
- Sigismund, S.; Confalonieri, S.; Ciliberto, A.; Polo, S.; Scita, G.; Di Fiore, P.P. Endocytosis and signaling: Cell logistics shape the eukaryotic cell plan. Physiol. Rev. 2012, 92, 273–366. [Google Scholar] [CrossRef]
- Conner, S.D.; Schmid, S.L. Regulated portals of entry into the cell. Nature 2003, 422, 37–44. [Google Scholar] [CrossRef]
- McMahon, H.T.; Boucrot, E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2011, 12, 517–533. [Google Scholar] [CrossRef]
- Cohen, A.W.; Hnasko, R.; Schubert, W.; Lisanti, M.P. Role of caveolae and caveolins in health and disease. Physiol. Rev. 2004, 84, 1341–1379. [Google Scholar] [CrossRef]
- Nabi, I.R.; Le, P.U. Caveolae/raft-dependent endocytosis. J. Cell Biol. 2003, 161, 673–677. [Google Scholar] [CrossRef]
- Le, P.U.; Guay, G.; Altschuler, Y.; Nabi, I.R. Caveolin-1 is a negative regulator of caveolae-mediated endocytosis to the endoplasmic reticulum. J. Biol. Chem. 2002, 277, 3371–3379. [Google Scholar] [CrossRef]
- Tachikawa, M.; Morone, N.; Senju, Y.; Sugiura, T.; Hanawa-Suetsugu, K.; Mochizuki, A.; Suetsugu, S. Measurement of caveolin-1 densities in the cell membrane for quantification of caveolar deformation after exposure to hypotonic membrane tension. Sci. Rep. 2017, 7, 7794. [Google Scholar] [CrossRef]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef]
- Vercauteren, D.; Vandenbroucke, R.E.; Jones, A.T.; Rejman, J.; Demeester, J.; De Smedt, S.C.; Sanders, N.N.; Braeckmans, K. The Use of Inhibitors to Study Endocytic Pathways of Gene Carriers: Optimization and Pitfalls. Mol. Ther. 2010, 18, 561–569. [Google Scholar] [CrossRef] [Green Version]
- Palozza, P.; Sestito, R.; Picci, N.; Lanza, P.; Monego, G.; Ranelletti, F.O. The sensitivity to beta-carotene growth-inhibitory and proapoptotic effects is regulated by caveolin-1 expression in human colon and prostate cancer cells. Carcinogenesis 2008, 29, 2153–2161. [Google Scholar] [CrossRef]
- Gai, X.; Lu, Z.; Tu, K.; Liang, Z.; Zheng, X. Caveolin-1 is up-regulated by GLI1 and contributes to GLI1-driven EMT in hepatocellular carcinoma. PLoS ONE 2014, 9, e84551. [Google Scholar] [CrossRef]
- Truong, T.Q.; Aubin, D.; Falstrault, L.; Brodeur, M.R.; Brissette, L. SR-BI, CD36, and caveolin-1 contribute positively to cholesterol efflux in hepatic cells. Cell Biochem. Funct. 2010, 28, 480–489. [Google Scholar] [CrossRef]
- Nagasawa, S.; Ogura, K.; Tsutsuki, H.; Saitoh, H.; Moss, J.; Iwase, H.; Noda, M.; Yahiro, K. Uptake of Shiga-toxigenic Escherichia coli SubAB by HeLa cells requires an actin- and lipid raft-dependent pathway. Cell Microbiol. 2014, 16, 1582–1601. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C-T method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]












| Cell Line | Caveolin-1 | GAPDH | Caveolin-2 | GAPDH |
|---|---|---|---|---|
| CACO-2 | 27.03 ± 0.06 | 17.51 ± 0.02 | 23.99 ± 0.07 | 17.55 ± 0.03 |
| HeLa | 21.15 ± 0.08 | 17.24 ± 0.04 | 2.32 ± 0.06 | 17.33 ± 0.02 |
| HepG2 | 35.69 ± 0.31 | 17.65 ± 0.02 | 34.89 ± 0.43 | 17.66 ± 0.03 |
| Huh7 | 25.92 ± 0.05 | 17.77 ± 0.02 | 24.33 ± 0.17 | 17.81 ± 0.02 |
| Cell Line | Intensity as % of Control |
|---|---|
| HeLa | 94 ± 6.9 |
| Huh7 | 170 ± 26 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phatvej, W.; Datta, H.K.; Wilkinson, S.C.; Mutch, E.; Daly, A.K.; Horrocks, B.R. Endocytosis and Lack of Cytotoxicity of Alkyl-Capped Silicon Quantum Dots Prepared from Porous Silicon. Materials 2019, 12, 1702. https://doi.org/10.3390/ma12101702
Phatvej W, Datta HK, Wilkinson SC, Mutch E, Daly AK, Horrocks BR. Endocytosis and Lack of Cytotoxicity of Alkyl-Capped Silicon Quantum Dots Prepared from Porous Silicon. Materials. 2019; 12(10):1702. https://doi.org/10.3390/ma12101702
Chicago/Turabian StylePhatvej, Wipaporn, Harish K. Datta, Simon C. Wilkinson, Elaine Mutch, Ann K. Daly, and Benjamin R. Horrocks. 2019. "Endocytosis and Lack of Cytotoxicity of Alkyl-Capped Silicon Quantum Dots Prepared from Porous Silicon" Materials 12, no. 10: 1702. https://doi.org/10.3390/ma12101702
APA StylePhatvej, W., Datta, H. K., Wilkinson, S. C., Mutch, E., Daly, A. K., & Horrocks, B. R. (2019). Endocytosis and Lack of Cytotoxicity of Alkyl-Capped Silicon Quantum Dots Prepared from Porous Silicon. Materials, 12(10), 1702. https://doi.org/10.3390/ma12101702