Lactoferrin-Hydroxyapatite Containing Spongy-Like Hydrogels for Bone Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Hydrogels
2.2. Physico-Chemical Characterization of Spongy-Like Hydrogels’
2.2.1. Fourier Transformed Infrared Spectroscopy (FTIR)
2.2.2. X-ray Diffraction (XRD) Analysis
2.2.3. Scanning Electron Microscopy
2.2.4. Micro Computed Tomography
2.2.5. Degradation Studies: Mass Loss
2.2.6. Water Uptake
2.2.7. Quantification of Lf Release: BCA Assay
2.3. In Vitro Studies
2.3.1. Cell Isolation and Culture
2.3.2. Cell Entrapment
2.3.3. Cell Viability
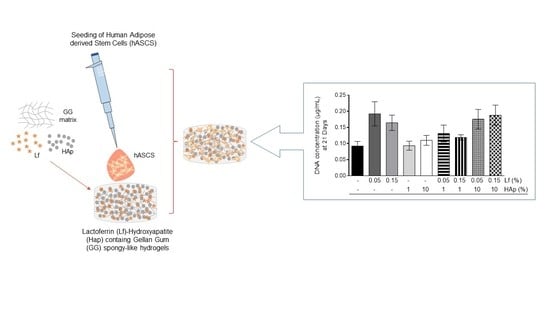
2.3.4. DNA Content
2.3.5. Cytoskeleton Morphology (Phalloidin/DAPI)
2.4. Statistics
3. Results
3.1. FTIR and XRD Analysis
3.2. SEM and Micro-CT Analysis
3.3. Water Uptake Analysis
3.4. Degradation Tests and Lf Release Analysis
3.5. Cell Viability and Cell Cytoskeleton Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef]
- Black, C.R.M.; Goriainov, V.; Gibbs, D.; Kanczler, J.; Tare, R.S.; Oreffo, R.O.C. Bone Tissue Engineering. Curr. Mol. Biol. Rep. 2015, 1, 132–140. [Google Scholar] [CrossRef]
- Shi, P.; Wang, Q.; Yu, C.; Fan, F.; Liu, M.; Tu, M.; Lu, W.; Du, M. Hydroxyapatite nanorod and microsphere functionalized with bioactive lactoferrin as a new biomaterial for enhancement bone regeneration. Colloids Surf. B Biointerfaces 2017, 155, 477–486. [Google Scholar] [CrossRef]
- Henkel, J.; Woodruff, M.A.; Epari, D.R.; Steck, R.; Glatt, V.; Dickinson, I.C.; Choong, P.F.M.; Schuetz, M.A.; Hutmacher, D.W. Bone Regeneration Based on Tissue Engineering Conceptions—A 21st Century Perspective. Bone Res. 2013, 1, 216–248. [Google Scholar] [CrossRef]
- Kumar, S.D. Study of Development and Applications of Bioactive Materials and Methods In Bone Tissue Engineering. Biomed. Res. 2015, 26, S55–S61. [Google Scholar]
- Silva, S.S.; Fernandes, E.M.; Silva-Correia, J.; Pina, S.C.A.; Vieira, S.; Oliveira, J.M.; Reis, R.L. Natural-Origin Materials for Tissue Engineering and Regenerative Medicine. In Comprehensive Biomaterials II, 2nd ed.; Ducheyne, P., Healy, K., Hutmacher, D.W., Grainger, D.W., Kirkpatrick, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Matassi, F.; Nistri, L.; Paez, D.C.; Innocenti, M. New biomaterials for bone regeneration. Clin. Cases Miner. Bone Metab. 2011, 8, 21–24. [Google Scholar]
- Lönnerdal, B. Lactoferrin: Molecular Structure and Biological Function. Annu. Rev. Nutr. 1995, 15, 93–110. [Google Scholar] [CrossRef]
- Farnaud, S.; Evans, R.W. Lactoferrin—A multifunctional protein with antimicrobial properties. Mol. Immunol. 2003, 40, 395–405. [Google Scholar] [CrossRef]
- Gao, R.; Watson, M.; Callon, K.E.; Tuari, D.; Dray, M.; Naot, D.; Amirapu, S.; Munro, J.T.; Cornish, J.; Musson, D.S. Local application of lactoferrin promotes bone regeneration in a rat critical-sized calvarial defect model as demonstrated by micro-CT and histological analysis. J. Tissue Eng. Regen. Med. 2018, 12, e620–e626. [Google Scholar] [CrossRef]
- Bruni, N.; Capucchio, M.T.; Biasibetti, E.; Pessione, E.; Cirrincione, S.; Giraudo, L.; Corona, A.; Dosio, F. Antimicrobial Activity of Lactoferrin-Related Peptides and Applications in Human and Veterinary Medicine. Molecules 2016, 21, 752. [Google Scholar] [CrossRef]
- Montesi, M.; Panseri, S.; Iafisco, M.; Adamiano, A.; Tampieri, A. Coupling Hydroxyapatite Nanocrystals with Lactoferrin as a Promising Strategy to Fine Regulate Bone Homeostasis. PLoS ONE 2015, 10, e0132633. [Google Scholar] [CrossRef]
- Jun, W.; Bi-Yu, Z.; Chuang-Yue, Z. Effect of lactoferrin on rat osteoblast proliferation. J. Hainan Med. Univ. 2016, 22, 5–8. [Google Scholar]
- Amini, A.A.; Nair, L.S. Recombinant human lactoferrin as a biomaterial for bone tissue engineering: Mechanism of antiapoptotic and osteogenic activity. Adv. Healthc. Mater. 2014, 3, 897–905. [Google Scholar] [CrossRef]
- Yoshimaki, T.; Sato, S.; Tsunori, K.; Shino, H.; Iguchi, S.; Arai, Y.; Ito, K.; Ogiso, B. Bone regeneration with systemic administration of lactoferrin in non-critical-sized rat calvarial bone defects. J. Oral Sci. 2013, 55, 343–348. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhu, S.; Hu, J. Bone Regeneration Is Promoted by Orally Administered Bovine Lactoferrin in a Rabbit Tibial Distraction Osteogenesis Model. Clin. Orthop. Relat. Res. 2015, 473, 2383–2393. [Google Scholar] [CrossRef] [Green Version]
- Takaoka, R.; Hikasa, Y.; Hayashi, K.; Tabata, Y. Bone Regeneration by Lactoferrin Released from a Gelatin Hydrogel. J. Biomater. Sci. Polym. Ed. 2011, 22, 1581–1589. [Google Scholar] [CrossRef] [Green Version]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Fabrication, Properties and Applications of Dense Hydroxyapatite: A Review. J. Funct. Biomater. 2015, 6, 1099–1140. [Google Scholar] [CrossRef] [Green Version]
- Tayyebi, S.; Mirjalili, F.; Samadi, H.; Nemati, A. A Review of Synthesis and Properties of Hydroxyapatite/Alumina Nano Composite Powder. Chem. J. 2015, 5, 20–28. [Google Scholar]
- Haider, A.; Haider, S.; Han, S.S.; Kang, I.-K. Recent advances in the synthesis, functionalization and biomedical applications of hydroxyapatite: A review. RSC Adv. 2017, 7, 7442–7458. [Google Scholar] [CrossRef]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite—Past, Present, and Future in Bone Regeneration. Bone Tissue Regen. Insights 2016, 7. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Maia, F.R.; Musson, D.S.; Naot, D.; Da Silva, L.P.; Bastos, A.R.; Costa, J.B.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L.; Cornish, J. Differentiation of osteoclast precursors on gellan gum-based spongy-like hydrogels for bone tissue engineering. Biomed. Mater. 2018, 13, 035012. [Google Scholar] [CrossRef]
- Manda, M.G.; da Silva, L.P.; Cerqueira, M.T.; Pereira, D.R.; Oliveira, M.B.; Mano, J.F.; Marques, A.P.; Oliveira, J.M.; Correlo, V.M.; Reis, R.L. Gellan gum-hydroxyapatite composite spongy-like hydrogels for bone tissue engineering. J. Biomed. Mater. Res. Part A 2018, 106, 479–490. [Google Scholar] [CrossRef]
- Gantar, A.; Da Silva, L.P.; Oliveira, J.M.; Marques, A.P.; Correlo, V.M.; Novak, S.; Reis, R.L. Nanoparticulate bioactive-glass-reinforced gellan-gum hydrogels for bone-tissue engineering. Mater. Sci. Eng. C 2014, 43, 27–36. [Google Scholar] [CrossRef]
- Cerqueira, M.T.; da Silva, L.P.; Correlo, V.M.; Reis, R.L.; Marques, A.P. Epidermis recreation in spongy-like hydrogels: New opportunities to explore epidermis-like analogues. Mater. Today 2015, 18, 468–469. [Google Scholar] [CrossRef]
- Da Silva, L.P.; Cerqueira, M.T.; Sousa, R.A.; Reis, R.L.; Correlo, V.M.; Marques, A.P. Engineering cell-adhesive gellan gum spongy-like hydrogels for regenerative medicine purposes. Acta Biomater. 2014, 10, 4787–4797. [Google Scholar] [CrossRef]
- Ohsugi, H.; Habuto, Y.; Honda, M.; Aizawa, M.; Kanzawa, N. Evaluation of the Anti-Bacterial Activity of a Novel Chelate-Setting Apatite Cement Containing Lactoferrin. Key Eng. Mater. 2013, 529, 187–191. [Google Scholar] [CrossRef]
- Kim, S.E.; Lee, D.-W.; Yun, Y.-P.; Shim, K.-S.; Jeon, D.I.; Rhee, J.-K.; Park, K. Heparin-immobilized hydroxyapatite nanoparticles as a lactoferrin delivery system for improving osteogenic differentiation of adipose-derived stem cells. Biomed. Mater. 2016, 11, 25004. [Google Scholar] [CrossRef]
- Dai, R.; Wang, Z.; Samanipour, R.; Koo, K.-I.; Kim, K. Adipose-Derived Stem Cells for Tissue Engineering and Regenerative Medicine Applications. Stem Cells Int. 2016, 2016, 6737345. [Google Scholar] [CrossRef]
- Calabrese, G.; Giuffrida, R.; Forte, S.; Fabbi, C.; Figallo, E.; Salvatorelli, L.; Memeo, L.; Parenti, R.; Gulisano, M.; Gulino, R. Human adipose-derived mesenchymal stem cells seeded into a collagen-hydroxyapatite scaffold promote bone augmentation after implantation in the mouse. Sci. Rep. 2017, 7, 7110. [Google Scholar] [CrossRef]
- Krishna, K.A.; Vishalakshi, B. Gellan gum-based novel composite hydrogel: Evaluation as adsorbent for cationic dyes. J. Appl. Polym. Sci. 2017, 134, 45527. [Google Scholar] [CrossRef]
- Anghel, L.; Erhan, R. Structural Aspects of Lactoferrin and Serum Transferrin Observed by FTIR Spectroscopy. Chem. J. Mold. 2018, 13, 111–116. [Google Scholar]
- Olimpio, R.M.C.; De Oliveira, M.; De Síbio, M.T.; Moretto, F.C.F.; Deprá, I.C.; Mathias, L.S.; Gonçalves, B.M.; Rodrigues, B.M.; Tilli, H.P.; Coscrato, V.E.; et al. Cell viability assessed in a reproducible model of human osteoblasts derived from human adipose-derived stem cells. PLoS ONE 2018, 13, e0194847. [Google Scholar] [CrossRef]
- Chang, H.-I.; Wang, Y. Cell Responses to Surface and Architecture of Tissue Engineering Scaffolds. In Regenerative Medicine and Tissue Engineering—Cells and Biomaterials; Eberli, P.D., Ed.; InTechOpen: London, UK, 2011. [Google Scholar] [Green Version]
- Deville, S.; Saiz, E.; Tomsia, A.P. Freeze casting of hydroxyapatite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 5480–5489. [Google Scholar] [CrossRef] [Green Version]
- Bobbert, F.S.L.; Zadpoor, A.A. Effects of bone substitute architecture and surface properties on cell response, angiogenesis, and structure of new bone. J. Mater. Chem. B 2017, 5, 6175–6192. [Google Scholar] [CrossRef] [Green Version]
- Ryan, A.J.; Gleeson, J.P.; Matsiko, A.; Thompson, E.M.; O’Brien, F.J. Effect of different hydroxyapatite incorporation methods on the structural and biological properties of porous collagen scaffolds for bone repair. J. Anat. 2015, 227, 732–745. [Google Scholar] [CrossRef]
- Dey, S.; Pal, S. Evaluation of Collagen-hydroxyapatite Scaffold for Bone Tissue Engineering. In IFMBE Proceedings, Proceedings of the 13th International Conference on Biomedical Engineering, Singapore, 3–6 December 2008; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Correlo, V.M.; Pinho, E.D.; Pashkuleva, I.; Bhattacharya, M.; Neves, N.M.; Reis, R.L. Water Absorption and Degradation Characteristics of Chitosan-Based Polyesters and Hydroxyapatite Composites. Macromol. Biosci. 2007, 7, 354–363. [Google Scholar] [CrossRef] [Green Version]
- Montesi, M.; Panseri, S.; Iafisco, M.; Adamiano, A.; Tampieri, A. Effect of hydroxyapatite nanocrystals functionalized with lactoferrin in osteogenic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2015, 103, 224–234. [Google Scholar] [CrossRef]
- Cabañas, M.V.; Peña, J.; Román, J.; Ramírez-Santillán, C.; Matesanz, M.C.; Feito, M.J.; Portolés, M.T.; Vallet-Regí, M. Design of tunable protein-releasing nanoapatite/hydrogel scaffolds for hard tissue engineering. Mater. Chem. Phys. 2014, 144, 409–417. [Google Scholar] [CrossRef]








| Name | GG % (w/v) | Lf % (w/v) | HAp % (w/v) |
|---|---|---|---|
| GG | 1.25 | - | - |
| GG/Low Lf | 1.25 | 0.05 | - |
| GG/High Lf | 1.25 | 0.15 | - |
| GG/Low HAp | 1.25 | - | 1.00 |
| GG/High HAp | 1.25 | - | 10.00 |
| GG/Low Lf/ Low HAp | 1.25 | 0.05 | 1.00 |
| GG/High Lf/Low HAp | 1.25 | 0.15 | 1.00 |
| GG/Low Lf/High HAp | 1.25 | 0.05 | 10.00 |
| GG/High Lf/High HAp | 1.25 | 0.15 | 10.00 |
| Name | Porosity (%) | Pore Size (µm) | Wall Thickness (µm) |
|---|---|---|---|
| GG | 86.5 ± 1.34 | 73.18 ± 12.92 | 12,83 ± 0.87 |
| GG/Low Lf | 86.01 ± 1.81 | 73.14 ± 5.37 | 13.21 ± 0.14 |
| GG/High Lf | 86.98 ± 1.97 | 68.85 ± 0.93 | 13.45 ± 1.31 |
| GG/Low HAp | 78.44 ± 1.37 | 55.23 ± 5.22 | 17.43 ± 0.21 |
| GG/High HAp | 64.45 ± 3.79 | 42.80 ± 5.73 | 20.17 ± 0.66 |
| GG/Low Lf/ Low HAp | 81.30 ± 0.64 | 63.30 ± 3.66 | 16.07 ± 0.42 |
| GG/High Lf/Low HAp | 84.14 ± 3.21 | 79.06 ± 15.31 | 16.48 ± 0.62 |
| GG/Low Lf/High HAp | 67.98 ± 0.55 | 59.96 ± 3.53 | 27.95 ± 1.66 |
| GG/High Lf/High HAp | 71.22 ± 2.96 | 59.74 ± 2.00 | 25.66 ± 1.72 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastos, A.R.; da Silva, L.P.; Maia, F.R.; Pina, S.; Rodrigues, T.; Sousa, F.; Oliveira, J.M.; Cornish, J.; Correlo, V.M.; Reis, R.L. Lactoferrin-Hydroxyapatite Containing Spongy-Like Hydrogels for Bone Tissue Engineering. Materials 2019, 12, 2074. https://doi.org/10.3390/ma12132074
Bastos AR, da Silva LP, Maia FR, Pina S, Rodrigues T, Sousa F, Oliveira JM, Cornish J, Correlo VM, Reis RL. Lactoferrin-Hydroxyapatite Containing Spongy-Like Hydrogels for Bone Tissue Engineering. Materials. 2019; 12(13):2074. https://doi.org/10.3390/ma12132074
Chicago/Turabian StyleBastos, Ana R., Lucília P. da Silva, F. Raquel Maia, Sandra Pina, Tânia Rodrigues, Filipa Sousa, Joaquim M. Oliveira, Jillian Cornish, Vitor M. Correlo, and Rui L. Reis. 2019. "Lactoferrin-Hydroxyapatite Containing Spongy-Like Hydrogels for Bone Tissue Engineering" Materials 12, no. 13: 2074. https://doi.org/10.3390/ma12132074
APA StyleBastos, A. R., da Silva, L. P., Maia, F. R., Pina, S., Rodrigues, T., Sousa, F., Oliveira, J. M., Cornish, J., Correlo, V. M., & Reis, R. L. (2019). Lactoferrin-Hydroxyapatite Containing Spongy-Like Hydrogels for Bone Tissue Engineering. Materials, 12(13), 2074. https://doi.org/10.3390/ma12132074