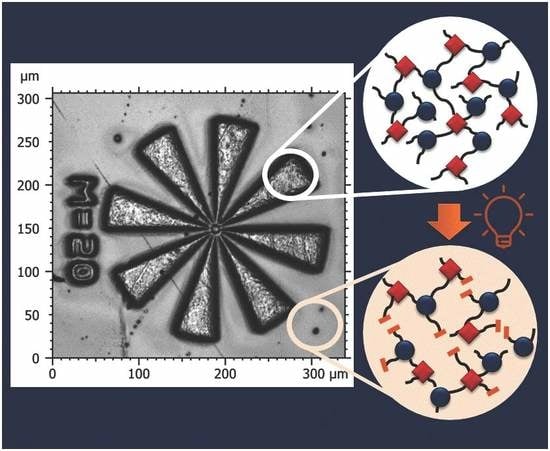
Photopatternable Epoxy-Based Thermosets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation and Thermal Curing of Photopatternable Epoxy-Based Thermosets
2.3. Characterization of Curing and Cleavage Kinetics
2.4. Characterization of Network Properties
2.5. Preparation and Characterization of Photopatterned Films
3. Results and Discussion
3.1. Thermal Curing of Photopatternable Epoxy-Based Thermosets
3.2. Photocleavage of Photopatternable Epoxy-Based Thermosets
3.3. Photopatterning Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ratna, D. Handbook of Thermoset Resins, 1st ed.; iSmithers: Shropshire, UK, 2009; ISBN 978-1-84735-410-5. [Google Scholar]
- Sangermano, M.; Pegel, S.; Pötschke, P.; Voit, B. Antistatic Epoxy Coatings with Carbon Nanotubes Obtained by Cationic Photopolymerization. Macromol. Rapid Commun. 2008, 29, 396–400. [Google Scholar] [CrossRef]
- Pleşa, I.; Noţingher, P.; Stancu, C.; Wiesbrock, F.; Schlögl, S. Polyethylene Nanocomposites for Power Cable Insulations. Polymers 2019, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.; Pavuluri, S.K.; Leonard, M.T.; Desmulliez, M.P.Y.; Arrighi, V. Microwave and thermal curing of an epoxy resin for microelectronic applications. Thermochim. Acta 2015, 616, 100–109. [Google Scholar] [CrossRef]
- Vidil, T.; Tournilhac, F.; Musso, S.; Robisson, A.; Leibler, L. Control of reactions and network structures of epoxy thermosets. Prog. Polym. Sci. 2016, 62, 126–179. [Google Scholar] [CrossRef] [Green Version]
- Ellis, B. Chemistry and Technology of Epoxy Resins; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Pleşa, I.; Noţingher, P.; Schlögl, S.; Sumereder, C.; Muhr, M. Properties of Polymer Composites Used in High-Voltage Applications. Polymers 2016, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Z.; Wei, Y.; Ji, Y. Gold Nanospheres Dispersed Light Responsive Epoxy Vitrimers. Polymers 2018, 10, 65. [Google Scholar] [CrossRef]
- Yang, Y.; Terentjev, E.M.; Wei, Y.; Ji, Y. Solvent-assisted programming of flat polymer sheets into reconfigurable and self-healing 3D structures. Nat. Commun. 2018, 9, 1906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radl, S.; Kreimer, M.; Griesser, T.; Oesterreicher, A.; Moser, A.; Kern, W.; Schlögl, S. New strategies towards reversible and mendable epoxy based materials employing [4πs+4πs] photocycloaddition and thermal cycloreversion of pendant anthracene groups. Polymer 2015, 80, 76–87. [Google Scholar] [CrossRef]
- Brutman, J.P.; Delgado, P.A.; Hillmyer, M.A. Polylactide Vitrimers. ACS Macro Lett. 2014, 3, 607–610. [Google Scholar] [CrossRef] [Green Version]
- Fortman, D.J.; Brutman, J.P.; Cramer, C.J.; Hillmyer, M.A.; Dichtel, W.R. Mechanically activated, catalyst-free polyhydroxyurethane vitrimers. J. Am. Chem. Soc. 2015, 137, 14019–14022. [Google Scholar] [CrossRef]
- Imbernon, L.; Norvez, S. From landfilling to vitrimer chemistry in rubber life cycle. Eur. Polym. J. 2016, 82, 347–376. [Google Scholar] [CrossRef]
- Bertrand, O.; Gohy, J.F. Photo-responsive polymers: Synthesis and applications. Polym. Chem. 2017, 8, 52–73. [Google Scholar] [CrossRef]
- Gohy, J.F.; Zhao, Y. Photo-responsive block copolymer micelles: Design and behavior. Chem. Soc. Rev. 2013, 42, 7117–7129. [Google Scholar] [CrossRef] [PubMed]
- Govan, J.M.; Young, D.D.; Lively, M.O.; Deiters, A. Optically trigged immune response through photocaged oligonucleotides. Tetrahedron Lett. 2015, 56, 3639–3642. [Google Scholar] [CrossRef] [PubMed]
- Takamori, S.; Yamaguchi, S.; Ohashi, N.; Nagamune, T. Sterically bulky caging for light-inducible protein activation. Chem. Commun. (Camb.) 2013, 49, 3013–3015. [Google Scholar] [CrossRef] [PubMed]
- Edler, M.; Mayrbrugger, S.; Fian, A.; Trimmel, G.; Radl, S.; Kern, W.; Griesser, T. Wavelength selective refractive index modulation in a ROMP derived polymer bearing phenyl- and ortho-nitrobenzyl ester groups. J. Mater. Chem. C 2013, 1, 3931. [Google Scholar] [CrossRef]
- Ryan, D.; Parviz, B.A.; Linder, V.; Semetey, V.; Sia, S.K.; Su, J.; Mrksich, M.; Whitesides, G.M. Patterning multiple aligned self-assembled monolayers using light. Langmuir 2004, 20, 9080–9088. [Google Scholar] [CrossRef] [PubMed]
- Doh, J.; Irvine, D.J. Photogenerated polyelectrolyte bilayers from an aqueous-processible photoresist for multicomponent protein patterning. J. Am. Chem. Soc. 2004, 126, 9170–9171. [Google Scholar] [CrossRef] [PubMed]
- Choi, O.J.; Chung, M.K.; Ryu, Y.M.; Lee, M.H. Synthesis and characterization of new positive photosensitive polyimide having photocleavable 4, 5-dimethoxy-2-nitrobenzyl (DMNB) groups. Polymer-Korea 2002, 26, 701–709. [Google Scholar]
- Taylor, P.G.; Lee, J.K.; Zakhidov, A.A.; Chatzichristidi, M.; Fong, H.H.; DeFranco, J.A.; Malliaras, G.G.; Ober, C.K. Orthogonal Patterning of PEDOT: PSS for Organic Electronics using Hydrofluoroether Solvents. Adv. Mater. 2009, 21, 2314–2317. [Google Scholar] [CrossRef]
- Marchl, M.; Edler, M.; Haase, A.; Fian, A.; Trimmel, G.; Griesser, T.; Stadlober, B.; Zojer, E. Tuning the threshold voltage in organic thin-film transistors by local channel doping using photoreactive interfacial layers. Adv. Mater. Weinheim 2010, 22, 5361–5365. [Google Scholar] [CrossRef] [PubMed]
- Willson, C.G. Approaches to the Design of Radiation-Sensitive Polymeric Imaging Systems with Improved Sensitivity and Resolution. J. Electrochem. Soc. 1986, 133, 181. [Google Scholar] [CrossRef]
- Wilkins, C.W. Lithographic Evaluation of an o-Nitrobenzyl Ester Based Deep U.V. Resist System. J. Electrochem. Soc. 1982, 129, 2552. [Google Scholar] [CrossRef]
- Kim, M.; Choi, J.C.; Jung, H.R.; Katz, J.S.; Kim, M.G.; Doh, J. Addressable micropatterning of multiple proteins and cells by microscope projection photolithography based on a protein friendly photoresist. Langmuir 2010, 26, 12112–12118. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Deng, X.X.; Li, Z.L.; Du, F.S.; Li, Z.C. Multifunctional Photodegradable Polymers for Reactive Micropatterns. Macromolecules 2014, 47, 4660–4667. [Google Scholar] [CrossRef]
- Radl, S.; Kreimer, M.; Manhart, J.; Griesser, T.; Moser, A.; Pinter, G.; Kalinka, G.; Kern, W.; Schlögl, S. Photocleavable epoxy based materials. Polymer 2015, 69, 159–168. [Google Scholar] [CrossRef]
- Radl, S.; Roppolo, I.; Pölzl, K.; Ast, M.; Spreitz, J.; Griesser, T.; Kern, W.; Schlögl, S.; Sangermano, M. Light triggered formation of photo-responsive epoxy based networks. Polymer 2017, 109, 349–357. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Gerbase, A.E.; Petzhold, C.L.; Costa, A.P.O. Dynamic mechanical and thermal behavior of epoxy resins based on soybean oil. J. Am. Oil Chem. Soc. 2002, 79, 797–802. [Google Scholar] [CrossRef]
- Hosseini Nejad, E.; van Melis, C.G.W.; Vermeer, T.J.; Koning, C.E.; Duchateau, R. Alternating Ring-Opening Polymerization of Cyclohexene Oxide and Anhydrides: Effect of Catalyst, Cocatalyst, and Anhydride Structure. Macromolecules 2012, 45, 1770–1776. [Google Scholar] [CrossRef]
- Liu, D.F.; Zhu, L.Q.; Wu, J.; Wu, L.Y.; Lü, X.Q. Ring-opening copolymerization of epoxides and anhydrides using manganese(iii) asymmetrical Schiff base complexes as catalysts. RSC Adv. 2015, 5, 3854–3859. [Google Scholar] [CrossRef]
- Pan, X.; Webster, D.C. Impact of structure and functionality of core polyol in highly functional biobased epoxy resins. Macromol. Rapid Commun. 2011, 32, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Pelliccioli, A.P.; Wirz, J. Photoremovable protecting groups: Reaction mechanisms and applications. Photochem. Photobiol. Sci. 2002, 1, 441–458. [Google Scholar] [CrossRef] [PubMed]
- Radl, S.V.; Schipfer, C.; Kaiser, S.; Moser, A.; Kaynak, B.; Kern, W.; Schlögl, S. Photo-responsive thiol–ene networks for the design of switchable polymer patterns. Polym. Chem. 2017, 8, 1562–1572. [Google Scholar] [CrossRef]
- Romano, A.; Roppolo, I.; Giebler, M.; Dietliker, K.; Možina, Š.; Šket, P.; Mühlbacher, I.; Schlögl, S.; Sangermano, M. Stimuli-responsive thiol-epoxy networks with photo-switchable bulk and surface properties. RSC Adv. 2018, 8, 41904–41914. [Google Scholar] [CrossRef] [Green Version]
- Giebler, M.; Radl, S.V.; Ast, M.; Kaiser, S.; Griesser, T.; Kern, W.; Schlögl, S. Dual-Responsive Polydimethylsiloxane Networks. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 2319–2329. [Google Scholar] [CrossRef]
- Rossegger, E.; Hennen, D.; Griesser, T.; Roppolo, I.; Schlögl, S. Directed motion of water droplets on multi-gradient photopolymer surfaces. Polym. Chem. 2019, 10, 1882–1893. [Google Scholar] [CrossRef]
- Völklein, F.; Zetterer, T. Praxiswissen Mikrosystemtechnik: Grundlagen—Technologien—Anwendungen; 2., vollständig überarbeitete und erweiterte Auflage; Springer Vieweg: Braunschweig, Germany, 2006; ISBN 9783834891051. [Google Scholar]







| Resin Formulation | Type of Anhydride | Molar Ratio 1 | Accelerator [wt%] |
|---|---|---|---|
| epoxy-NBE/HHMPA | HHMPA | 1 | 0.1 |
| epoxy-NBE/HHPA | HHPA | 1 | 0.1 |
| epoxy-NBE/DDSA | DDSA | 1 | 0.1 |
| epoxy-NBE/GA | GA | 1 | 0.1 |
| Resin Formulation | Tg [°C] | HIT [MPa] | Tg [°C] | HIT [MPa] |
|---|---|---|---|---|
| Thermally Cured | Photocleaved | |||
| epoxy-NBE/HHMPA | 72 | 213.5 ± 19.8 | 48 | 110.9 ± 10.3 |
| epoxy-NBE/HHPA | 46 | 148.6 ± 13.8 | 32 | 83.0 ± 6.3 |
| epoxy-NBE/DDSA | 44 | 51.0 ± 4.7 | 30 | 41.3 ± 3.8 |
| epoxy-NBE/GA | 16 | 8.6 ± 0.8 | 12 | 1.3 ± 0.1 |
| Resin Formulation | D0 [J/cm2] | D100 [J/cm2] | γ | Resolution [µm] |
|---|---|---|---|---|
| epoxy-NBE/HHMPA | 16.3 | 2.27 | 1.17 | 8 |
| epoxy-NBE/HHPA | 19.5 | 0.26 | 0.53 | >10 |
| epoxy-NBE/DDSA | 19.7 | 0.25 | 0.53 | >10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giebler, M.; Radl, S.; Ules, T.; Griesser, T.; Schlögl, S. Photopatternable Epoxy-Based Thermosets. Materials 2019, 12, 2350. https://doi.org/10.3390/ma12152350
Giebler M, Radl S, Ules T, Griesser T, Schlögl S. Photopatternable Epoxy-Based Thermosets. Materials. 2019; 12(15):2350. https://doi.org/10.3390/ma12152350
Chicago/Turabian StyleGiebler, Michael, Simone Radl, Thomas Ules, Thomas Griesser, and Sandra Schlögl. 2019. "Photopatternable Epoxy-Based Thermosets" Materials 12, no. 15: 2350. https://doi.org/10.3390/ma12152350
APA StyleGiebler, M., Radl, S., Ules, T., Griesser, T., & Schlögl, S. (2019). Photopatternable Epoxy-Based Thermosets. Materials, 12(15), 2350. https://doi.org/10.3390/ma12152350