Increased Sustainability of Carbon Dioxide Mineral Sequestration by a Technology Involving Fly Ash Stabilization
Abstract
:1. Introduction
- a)
- The carbonation reactions are by-products of a stabilization procedure, based on the use of amorphous silica as leachable heavy metals stabilizer, that also involve carbon dioxide sequestration;
- b)
- All the materials used in the process are wastes and by-products;
- c)
- The reactions do not require control of temperature or pressure conditions. Indeed, it is fundamental to highlight that there are several studies focused on accelerated carbonation of MSW residues. In the majority of these works, accelerated carbonation tests were performed on humidified samples, applying pressures of CO2 and thermal treatments;
- d)
- The process is realized in the frame of “Azure chemistry” approach [20]. Then its sustainability is guarantee by the approach fundamentals;
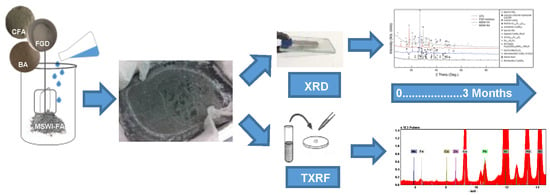
2. Materials and Methods
3. Results
4. Conclusions
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Rahmani, O. CO2 sequestration by indirect mineral carbonation of Ind. waste red gypsum. J. CO2 Util. 2018, 27, 374–380. [Google Scholar] [CrossRef]
- Kaliyavaradhan, S.K.; Ling, T.-C. Potential of CO2 sequestration through construction and demolition (C&D) waste—An overview. J. CO2 Util. 2017, 20, 234–242. [Google Scholar]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef] [PubMed]
- Saran, R.K.; Arora, V.; Yadav, S. Copyright© 2018 Global NEST Printed in Greece. All rights reserved. CO2 Glob. NEST J. 2018, 20, 497–503. [Google Scholar]
- Rahmani, O.; Kadkhodaie, A.; Highfield, J. Kinetics Analysis of CO2 Mineral Carbonation Using Byproduct Red Gypsum. Energy Fuels 2016, 30, 7460–7464. [Google Scholar] [CrossRef]
- Wang, T.; Huang, H.; Hu, X.; Fang, M.; Luo, Z.; Guo, R. Accelerated mineral carbonation curing of cement paste for CO2 sequestration and enhanced properties of blended calcium silicate. Chem. Eng. J. 2017, 323, 320–329. [Google Scholar] [CrossRef]
- McCutcheon, J.; Power, I.M.; Shuster, J.; Harrison, A.L.; Dipple, G.M.; Southam, G. Carbon Sequestration in Biogenic Magnesite and Other Magnesium Carbonate Minerals. Environ. Sci. Technol. 2019, 53, 3225–3237. [Google Scholar] [CrossRef]
- Gadikota, G.; Matter, J.; Kelemen, P.; Park, A.A. Chemical and morphological changes during olivine carbonation for CO2 storage in the presence of NaCl and NaHCO3. Phys. Chem. Chem. Phys. 2014, 16, 4679. [Google Scholar] [CrossRef]
- Wee, J.-H. A review on carbon dioxide capture and storage technology using coal fly ash. Appl. Energy 2013, 106, 143–151. [Google Scholar] [CrossRef]
- Huntzinger, D.N.; Gierke, J.S.; Sutter, L.L.; Kawatra, S.K.; Eisele, T.C. Mineral carbonation for carbon sequestration in cement kiln dust from waste piles. J. Hazard. Mater. 2009, 168, 31–37. [Google Scholar] [CrossRef]
- Siriwardena, D.P.; Peethamparan, S. Quantification of CO2 sequestration capacity and carbonation rate of alkaline industrial byproducts. Constr. Build. Mater. 2015, 91, 216–224. [Google Scholar] [CrossRef]
- Baciocchi, R.; Costa, G.; Polettini, A.; Pomi, R.; Stramazzo, A.; Zingaretti, D. Accelerated Carbonation of Steel Slags Using CO2 Diluted Sources: CO2 Uptakes and Energy Requirements. Front. Energy Res. 2016, 3, 56. [Google Scholar] [CrossRef]
- Harrison, A.L.; Power, I.M.; Dipple, G.M. Accelerated Carbonation of Brucite in Mine Tailings for Carbon Sequestration. Environ. Sci. Technol. 2013, 47, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Mastali, M.; Abdollahnejad, Z. Carbon dioxide sequestration on fly ash/waste glassalkali-based mortars with recycled aggregates: Compressive strength, hydration products, carbon footprint, and cost analysis. In Carbon Dioxide Sequestration Cementitious Construction Materials, 1st ed.; Woodhead Publishing: Cambridge, UK, 2018; pp. 299–348. [Google Scholar]
- Mastali, M.; Abdollahnejad, Z.; Pacheco-Torgal, F. Performance of waste based alkaline mortars submitted to accelerated carbon dioxide curing. Resour. Conserv. Recycl. 2018, 129, 12–19. [Google Scholar] [CrossRef]
- Rendek, E.; Ducom, G.; Germain, P. Carbon dioxide sequestration in municipal solid waste incinerator (MSWI) bottom ash. J. Hazard. Mater. 2006, 128, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Quina, M.J.; Bontempi, E.; Bogush, A.; Schlumberger, S.; Weibel, G.; Braga, R.; Funari, V.; Hyks, J.; Rasmussen, E.; Lederer, J. Technologies for the management of MSW incineration ashes from gas cleaning: New perspectives on recovery of secondary raw materials and circular economy. Sci. Total Environ. 2018, 635, 526–542. [Google Scholar] [CrossRef]
- Struis, R.P.W.J.; Pasquali, M.; Borgese, L.; Gianoncelli, A.; Gelfi, M.; Colombi, P.; Thiaudière, D.; Depero, L.E.; Rizzo, G.; Bontempi, E. Inertisation of heavy metals in municipal solid waste incineration fly ash by means of colloidal silica—A synchrotron X-ray diffraction and absorption study. RSC Adv. 2013, 3, 14339. [Google Scholar] [CrossRef]
- Bontempi, E. Raw Materials Substitution Sustainability. In SpringerBriefs in Applied Sciences and Technology; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Zanoletti, A.; Bilo, F.; Depero, L.E.; Zappa, D.; Bontempi, E. The first sustainable material designed for air particulate matter capture: An introduction to Azure Chemistry. J. Environ. Manag. 2018, 218, 355–362. [Google Scholar] [CrossRef]
- Benassi, L.; Zanoletti, A.; Depero, L.E.; Bontempi, E. Sewage sludge ash recovery as valuable raw material for chemical stabilization of leachable heavy metals. J. Environ. Manag. 2019, 245, 464–470. [Google Scholar] [CrossRef]
- Bontempi, E. A new approach for evaluating the sustainability of raw materials substitution based on embodied energy and the CO2 footprint. J. Clean. Prod. 2017, 162, 162–169. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Comans, R.N.J. Mineral CO2 sequestration by carbonation of industrial residues. In Literature Review and Selection of Residue; No. ECN-C--05-074; Energy research Centre of the Netherlands ECN: Sint Maartensvlotbrug, Netherlands, 2005. [Google Scholar]
- Teir, S.; Eloneva, S.; Fogelholm, C.-J.; Zevenhoven, R. Fixation of carbon dioxide by producing hydromagnesite from serpentinite. Appl. Energy 2009, 86, 214–218. [Google Scholar] [CrossRef]
- Pasquali, M.; Zanoletti, A.; Benassi, L.; Federici, S.; Depero, L.E.; Bontempi, E. Stabilized biomass ash as a sustainable substitute for commercial P-fertilizers. L. Degrad. Dev. 2018, 29, 2199–2207. [Google Scholar] [CrossRef]
- Liu, M.; Gadikota, G. Phase Evolution and Textural Changes during the Direct Conversion and Storage of CO2 to Produce Calcium Carbonate from Calcium Hydroxide. Geosciences 2018, 8, 445. [Google Scholar] [CrossRef]
- Bontempi, E.; Zacco, A.; Borgese, L.; Gianoncelli, A.; Ardesi, R.; Depero, L.E. A new method for municipal solid waste incinerator (MSWI) fly ash inertization, based on colloidal silica. J. Environ. Monit. 2010, 12, 2093–2099. [Google Scholar] [CrossRef] [PubMed]
- Rodella, N.; Bosio, A.; Dalipi, R.; Zacco, A.; Borgese, L.; Depero, L.E.; Bontempi, E. Waste silica sources as heavy metal stabilizers for municipal solid waste incineration fly ash. Arab. J. Chem. 2017, 10, S3676–S3681. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Fahimi, A.; Bilo, F.; Dalipi, R.; Assi, A.; Federici, S.; Guedes, A.; Valentim, B.; Bontempi, E. Raw materials and sustainability: Could poultry litter ash be used as heavy metals stabilizer instead of other resources? J. Waste Manag. under review.
- Benassi, L.; Pasquali, M.; Zanoletti, A.; Dalipi, R.; Borgese, L.; Depero, L.E.; Vassura, I.; Quina, M.J.; Bontempi, E. Chemical Stabilization of Municipal Solid Waste Incineration Fly Ash without Any Commercial Chemicals: First Pilot-Plant Scaling Up. ACS Sustain. Chem. Eng. 2016, 4, 5561–5569. [Google Scholar] [CrossRef]
- Assi, A.; Bilo, F.; Zanoletti, A.; Ponti, J.; Valsesia, A.; La Spina, R.; Zacco, A.; Bontempi, E.; Zacco, E.B. Urban mining of waste-to-energy residues for a zero-waste approach in municipal solid waste incineration. J. Clean. Prod. under review.
- Bosio, A.; Zacco, A.; Borgese, L.; Rodella, N.; Colombi, P.; Benassi, L.; Depero, L.E.; Bontempi, E. A sustainable technology for Pb and Zn stabilization based on the use of only waste materials: A green chemistry approach to avoid chemicals and promote CO2 sequestration. Chem. Eng. J. 2014, 253, 377–384. [Google Scholar] [CrossRef]
- Benassi, L.; Dalipi, R.; Consigli, V.; Pasquali, M.; Borgese, L.; Depero, L.E.; Clegg, F.; Bingham, P.A.; Bontempi, E. Integrated management of ash from industrial and domestic combustion: A new sustainable approach for reducing greenhouse gas emissions from energy conversion. Environ. Sci. Pollut. Res. 2017, 24, 14834–14846. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Shimaoka, T.; Saffarzadeh, A.; Takahashi, F. Mineralogical characterization of municipal solid waste incineration bottom ash with an emphasis on heavy metal-bearing phases. J. Hazard. Mater. 2011, 187, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Choi, D.; Kim, M.H.; Park, Y. Tuning Crystal Polymorphisms and Structural Investigation of Precipitated Calcium Carbonates for CO2 Mineralization. ACS Sustain. Chem. Eng. 2017, 5, 1659–1667. [Google Scholar] [CrossRef]
- Tian, S.; Jiang, J. Sequestration of Flue Gas CO2 by Direct Gas–Solid Carbonation of Air Pollution Control System Residues. Environ. Sci. Technol. 2012, 46, 13545–13551. [Google Scholar] [CrossRef]
- Liu, W.; Su, S.; Xu, K.; Chen, Q.; Xu, J.; Sun, Z.; Wang, Y.; Hu, S.; Wang, X.; Xue, Y.; et al. CO2 sequestration by direct gas–solid carbonation of fly ash with steam addition. J. Clean. Prod. 2018, 178, 98–107. [Google Scholar] [CrossRef]
- Sarkar, A.; Mahapatra, S. Synthesis of All Crystalline Phases of Anhydrous Calcium Carbonate. Cryst. Growth Des. 2010, 10, 2129–2135. [Google Scholar] [CrossRef]




| Samples | MSWI FA (g) | CFA (g) | FGD (g) | Silica Fume (g) | MSWI-BA (g) | %MSWI FA (%) | %FGD (%) |
|---|---|---|---|---|---|---|---|
| A | 130 | 30 | 40 | 20 | - | 59.1 | 18.2 |
| B | 130 | 30 | - | - | 20 | 72.2 | - |
| C | 130 | 30 | 40 | - | 20 | 59.1 | 18.2 |
| D | 130 | 30 | 40 | - | - | 65 | 20 |
| Samples | pH | Months | Elemental Concentration (mg/L) | |||||
|---|---|---|---|---|---|---|---|---|
| Zn | Pb | |||||||
| MWSI-FA | 12.18 | - | 8.80 | ± | 4.30 | 34.60 | ± | 2.40 |
| MWSI-BA | 10.69 | - | 0.05 | ± | 0.04 | 0.09 | ± | 0.05 |
| CFA | 11.81 | - | 0.24 | ± | 0.02 | 0.13 | ± | 0.03 |
| FGD | 12.68 | - | 0.1 | ± | 0.04 | <LOD | ||
| A | 11.73 | 1 | 0.15 | ± | 0.09 | <LOD | ||
| 8.92 | 2 | 0.11 | ± | 0.01 | <LOD | |||
| B | 12.22 | 1 | 1.52 | ± | 0.19 | 12.20 | ± | 0.70 |
| 10.23 | 2 | 0.14 | ± | 0.00 | <LOD | |||
| C | 12.06 | 1 | 0.39 | ± | 0.02 | 3.20 | ± | 0.50 |
| 10.37 | 2 | 0.07 | ± | 0.01 | <LOD | |||
| D | 12.22 | 1 | 0.80 | ± | 0.40 | 6.40 | ± | 1.60 |
| 11.07 | 2 | 0.07 | ± | 0.001 | <LOD | |||
| Samples | Months | Amorphous (%) | Calcite (%) | Hannebachite (%) | Thaumasite (%) | Gypsum (%) | Quartz (%) | Vaterite (%) | Sylvite (%) | Halite (%) | Anhydrite (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample A | 0 | 6 * | |||||||||
| 1 | 69 | 12 | 5 | <1 | 1 | 1 | 3 | 2 | 3 | 4 | |
| 1.5 | 69 | 17 | 4 | <1 | 3 | <1 | 2 | 1 | 2 | 2 | |
| 2 | 67 | 17 | 5 | <1 | <1 | 1 | 1 | 2 | 2 | 5 | |
| 2.5 | 63 | 19 | 5 | <1 | <1 | 1 | 2 | 1 | 3 | 5 | |
| 3 | 63 | 20 | 4 | <1 | 3 | 2 | 2 | 1 | 2 | 3 | |
| Sample B | 0 | 11* | |||||||||
| 1 | 72 | 14 | 2 | <1 | 1 | 2 | 2 | 2 | 4 | <1 | |
| 1.5 | 70 | 16 | <1 | 3 | 1 | 2 | 3 | 1 | 2 | <1 | |
| 2 | 67 | 17 | <1 | <1 | 1 | 3 | 6 | <1 | 2 | 3 | |
| 2.5 | 67 | 17 | <1 | <1 | 1 | 2 | 4 | 1 | 2 | 4 | |
| 3 | 64 | 19 | 3 | <1 | 2 | <1 | 1 | 1 | 3 | 4 | |
| Sample C | 0 | 9* | |||||||||
| 1 | 69 | 13 | 6 | <1 | 1 | <1 | 2 | 2 | 3 | 3 | |
| 1.5 | 70 | 16 | 5 | <1 | 2 | 1 | 2 | 1 | 2 | 2 | |
| 2 | 69 | 16 | 4 | <1 | 2 | 1 | 2 | 1 | 1 | 3 | |
| 2.5 | 63 | 19 | 4 | <1 | 1 | 2 | 2 | 1 | 2 | 6 | |
| 3 | 64. | 20 | 3 | <1 | 1 | <1 | 3 | 1 | 1 | 5 | |
| Sample D | 0 | 7* | |||||||||
| 1 | 66 | 14 | 7 | <1 | <1 | <1 | 2 | 2. | 3 | 4 | |
| 1.5 | 65 | 21 | 6 | <1 | 1 | 1 | 2 | 1 | 2 | 1 | |
| 2 | 65 | 18 | 6 | <1 | 2 | 1 | 2 | <1 | 2 | 3 | |
| 2.5 | 63 | 22 | 5 | <1 | <1 | 2 | 3 | <1 | 2 | 2 | |
| 3 | 61 | 20 | 4 | <1 | 2 | 3 | 2 | 1 | 2 | 5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assi, A.; Federici, S.; Bilo, F.; Zacco, A.; Depero, L.E.; Bontempi, E. Increased Sustainability of Carbon Dioxide Mineral Sequestration by a Technology Involving Fly Ash Stabilization. Materials 2019, 12, 2714. https://doi.org/10.3390/ma12172714
Assi A, Federici S, Bilo F, Zacco A, Depero LE, Bontempi E. Increased Sustainability of Carbon Dioxide Mineral Sequestration by a Technology Involving Fly Ash Stabilization. Materials. 2019; 12(17):2714. https://doi.org/10.3390/ma12172714
Chicago/Turabian StyleAssi, Ahmad, Stefania Federici, Fabjola Bilo, Annalisa Zacco, Laura E. Depero, and Elza Bontempi. 2019. "Increased Sustainability of Carbon Dioxide Mineral Sequestration by a Technology Involving Fly Ash Stabilization" Materials 12, no. 17: 2714. https://doi.org/10.3390/ma12172714
APA StyleAssi, A., Federici, S., Bilo, F., Zacco, A., Depero, L. E., & Bontempi, E. (2019). Increased Sustainability of Carbon Dioxide Mineral Sequestration by a Technology Involving Fly Ash Stabilization. Materials, 12(17), 2714. https://doi.org/10.3390/ma12172714