Performance of PATC-PDMDAAC Composite Coagulants in Low-Temperature and Low-Turbidity Water Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
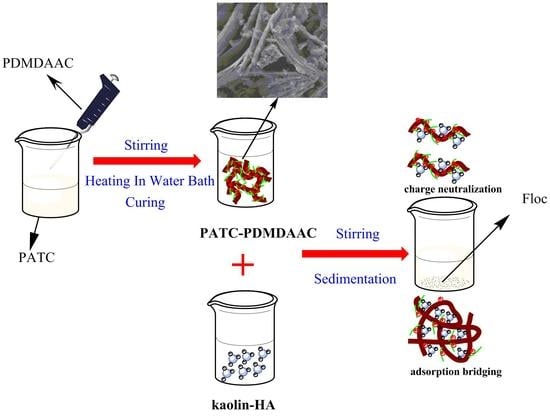
2.2. Preparation of PATC-PDMDAAC
2.3. Characterization of the Flocculant
2.4. Performance
3. Results and Discussion
3.1. Characterization
3.1.1. FTIR Spectra Analysis
3.1.2. XRD Analysis
3.1.3. SEM-EDS Analysis
3.1.4. TG/DSC Analysis
3.1.5. Morphological Analysis of Aluminum
3.2. Flocculation Properties
3.2.1. Effect of dosage
3.2.2. Effect of Different m(PDMDAAC)/m(PATC)
3.2.3. Effect of pH
3.2.4. Effect of Sedimentation Time
3.2.5. Effect of Stirring Speed
3.3. ZP Analysis
3.4. 3D Fluorescence Spectroscopy Analysis
3.5. Kinetic Investigation
3.6. Comparison of Actual Water Treatment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Morris, J.K.; Knocke, W.R. Temperature Effects on the Use of Metal-Ion Coagulants for Water Treatment. J. Am. Water Works Assoc. 1984, 76, 74–79. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, K.; Zhang, P.; Zeng, G. Pilot Study of Low-Temperature Low-Turbidity Reservoir Water Treatment Using Dual-Media Filtration with Micro-Flocculation. In Proceedings of the Paper presented at the International Conference on Multimedia Technology, Hangzhou, China, 26–28 July 2011. [Google Scholar]
- Duan, J.; Gregory, J. Coagulation by Hydrolysing Metal Salts. Adv. Colloid Interface Sci. 2003, 100, 475–502. [Google Scholar] [CrossRef]
- Parthasarathy, N.; Buffle, J. Study of Polymeric aluminium(III) Hydroxide Solutions for Application in Waste Water Treatment. Properties of the Polymer and Optimal Conditions of Preparation. Water Res. 1985, 19, 25–36. [Google Scholar] [CrossRef]
- Yan, M.; Wang, D.; Qu, J.; Ni, J.; Chow, C.W.K. Enhanced Coagulation for High Alkalinity and Micro-Polluted Water: The Third Way through Coagulant Optimization. Water Res. 2008, 42, 2278–2286. [Google Scholar] [CrossRef] [PubMed]
- Upton, W.V.; Buswell, A.M. Titanium Salts in Water Purification. Ind. Eng. Chem. 1937, 29, 870–871. [Google Scholar] [CrossRef]
- Lokshin, E.P.; Belikov, M.L. Water Purification with Titanium Compounds to Remove Fluoride Ions. Russ. J. Appl. Chem. 2003, 76, 1466–1471. [Google Scholar] [CrossRef]
- Xin, H.; Gao, B.; Yue, Q.; Yan, W.; Qian, L. Effect of Si/Ti Molar Ratio on Enhanced Coagulation Performance, Floc Properties and Sludge Reuse of a Novel Hybrid Coagulant: Polysilicate Titanium Sulfate. Desalination 2014, 352, 150–157. [Google Scholar]
- Song, M.; Liu, H.; Liu, J.; Huang, Q.; Song, W. Structure and performance of composite flocculant poly-tanium-aluminum-silicate. Chin. J. Environ. Eng. 2012, 6, 2661–2665. [Google Scholar]
- Xu, J.; Zhang, X.; Ma, Y.G. Treatment of Low-Temperature and Low-Turbidity Water Using Modified Active Silica Acid. Adv. Mater. Res. 2011, 374, 831–835. [Google Scholar] [CrossRef]
- Lou, I.; Gong, S.; Huang, X.; Liu, Y. Coagulation Optimization for Low Temperature and Low Turbidity Source Water Using Combined Coagulants: A Case Study. Desalin. Water Treat. 2012, 46, 107–114. [Google Scholar] [CrossRef]
- Zhang, Z.; Jing, R.; Shuran, H.E.; Qian, J.; Zhang, K.; Guilin, M.A.; Chang, X.; Zhang, M.; Yongtao, L.I. Coagulation of Low Temperature and Low Turbidity Water: Adjusting Basicity of Polyaluminum Chloride (PAC) and Using Chitosan as Coagulant Aid. Sep. Purif. Technol. 2018, 206, 131–139. [Google Scholar] [CrossRef]
- Butler, G.B.; Ingley, F.L. Preparation and Polymerization of Unsaturated Quaternary Ammonium Compounds. II. Halogenated Allyl Derivatives1,2. J. Am. Chem. Soc. 1951, 73, 895–896. [Google Scholar] [CrossRef]
- Lu, L. Preparation and Applications of the Composite Coagulant PAC-PDMDAAC. Master’s Thesis, Chongqing Universty, Chongqing, China, 2012. [Google Scholar]
- Zhang, Y.; Xu, J. Synthesis of Ultra High Molecular Weight Poly(dimethyldiallyl Ammonium Chloride). Russ. J. Appl. Chem. 2016, 89, 315–323. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, S.; Peng, Y.; Wang, J.; Peng, G. Preparation and Swelling Behavior of Amphoteric Superabsorbent Composite with semi-IPN Composed of Poly(Acrylic acid)/Ca-bentonite/poly(dimethyldiallylammonium Chloride). Polym. Adv. Technol. 2007, 18, 194–199. [Google Scholar] [CrossRef]
- Gong, Z.; Liu, L.; Zheng, Y. Study on preparation and application of poly(dimethyldiallylammonium chloride)-polyferric sulfate composite flocculant. Tech. Equip. Environ. Pollut. Control 2004, 5, 35–39. [Google Scholar]
- Liao, L.; Zhang, P. Preparation and Characterization of Polyaluminum Titanium Silicate and its Performance in the Treatment of Low-Turbidity Water. Processes 2018, 6, 125. [Google Scholar] [CrossRef]
- Jiang, S.; Li, X.; Feng, X. Synthesis and Characterization of PAC-PDMDAAC Organic-Inorganic Hybrid Polymer Flocculants. Acta Polym. Sin. 2014, 30, 466–472. [Google Scholar]
- Yang, J. Preparation and Applications Study of PDMDAAC. Master’s Thesis, Chongqing Universty, Chongqing, China, 2012. [Google Scholar]
- Francis, S.; Varshney, L.; Sabharwal, S. Thermal Degradation Behavior of Radiation Synthesized Polydiallyldimethylammonium Chloride. Eur. Polym. J. 2007, 43, 2525–2531. [Google Scholar] [CrossRef]
- Peng, J.; Xue, A.; Zhao, H.; Du, L. Al Species Distribution of Organic Silicate Aluminum Hybrid Flocculants by Al-Ferron Complexation Timed Spectrophotometric Method. Environ. Sci. 2008, 29, 2513–2517. [Google Scholar]
- Feng, L.; Luan, Z.; Tang, H. The Study on Speciation Analysis Methods for Hydrolysis Polymerization of Aluminum. Environ. Chem. 1993, 12, 373–379. [Google Scholar]
- Li, X.; Zhang, Y.; Zhao, X.; Sun, B.; Yi, X. Coagulation aid effect of PDM in treatment of winter yangtze river water combined with PFS. Ind. Water Wastewater 2009, 40, 19–22. [Google Scholar]
- Wang, X. Synthesis, Characterization and Application of organic-inorganic hybrid flocculant PAM-PAFC. Master’s Thesis, Chongqing Universty, Chongqing, China, 2018. [Google Scholar]
- Lancaster, J.E.; Baccei, L.; Panzer, H.P. The Structure of Poly(Diallyldimethyl-Ammonium) Chloride by 13C-NMR Spectroscopy. J. Polym. Sci. Polym. Chem. 1976, 14, 549–554. [Google Scholar] [CrossRef]
- Liu, L.; Gong, Z.; Huang, J.; Zheng, Y. Viscosity Behavior of Poly (dimethyldiallylammonium chloride) in Polyferric Sulfate. Chin. J. Inorg. Chem. 2004, 21, 1029–1033. [Google Scholar]
- Liu, Z.; Jiang, J.; Lie, F.; Wang, Q.; Sun, T. Preparation and coagulation performance of composite flocculant of PFMS-PDMDAAC. Chin. J. Environ. Eng. 2012, 6, 2183–2188. [Google Scholar]
- Chao, H.; Runhu, Z.; Li, L.; Xiaoying, Z. Adsorption of Phenol from Aqueous Solutions Using Activated Carbon Prepared from Crofton Weed. Desalin. Water Treat. 2012, 37, 230–237. [Google Scholar]
- Dong, Y.; Wang, T.; Wang, X.; He, D. Treatment of Printing and Dyeing Wastewater by Rare Eart Compound Sorbent Flocculant. Environ. Sci. Technol. 2015, 38, 152–156. [Google Scholar]
- Sun, C. Coagulation Performance and Flocs Properties of the Composite Flocculants Combining Polymeric Aluminum Ferric Chloride with Quanternary Ammonium Polymer. Ph.D. Thesis, Shandong University, Jinan, China, 2012. [Google Scholar]
- Huang, X.; Gao, B.; Sun, Y.; Yue, Q.; Wang, Y.; Li, Q.; Xu, X. Effects of Epichlorohydrin–Dimethylamine on Polytitanium Chloride Coagulation and Membrane Fouling in Humic-Kaolin Water Treatment: Dosage, Dose Method and Solution pH. Sep. Purif. Technol. 2017, 173, 209–217. [Google Scholar] [CrossRef]
- Klučáková, M.; Pekař, M. Solubility and Dissociation of Lignitic Humic Acids in Water Suspension. Colloid Surf. A 2005, 252, 157–163. [Google Scholar] [CrossRef]
- Wei, L.M.; Tang, T.; Huang, B.T. Synthesis and Characterization of Polyethylene/Clay-Silica Nanocomposites: A Montmorillonite/Silica-Hybrid-Supported Catalyst and in Situ Polymerization. J. Polym. Sci. Polym. Chem. 2004, 42, 941–949. [Google Scholar] [CrossRef]
- Ma, J.; Fu, K.; Fu, X.; Guan, Q.; Ding, L.; Shi, J.; Zhu, G.; Zhang, X.; Zhang, S.; Jiang, L. Flocculation Properties and Kinetic Investigation of Polyacrylamide with Different Cationic Monomer Content for High Turbid Water Purification. Sep. Purif. Technol. 2017, 182, 134–143. [Google Scholar] [CrossRef]
- Henderson, R.K.; Baker, A.; Murphy, K.R.; Hambly, A.; Stuetz, R.M.; Khan, S.J. Fluorescence as a Potential Monitoring Tool for Recycled Water Systems: A Review. Water Res. 2009, 43, 863–881. [Google Scholar] [CrossRef] [PubMed]
- Saar, R.A.; Weber, J.H. Comparison of Spectrofluorometry and Ion-Selective Electrode Potentiometry for Determination of Complexes Between Fulvic Acid and Heavy-Metal Ions. Anal. Chem. 1980, 52, 2095–2100. [Google Scholar] [CrossRef]
- Kaith, B.S.; Jindal, R.; Sharma, R. Study of Ionic Charge Dependent Salt Resistant Swelling Behavior and Removal of Colloidal Particles Using Reduced Gum Rosin-Poly(Acrylamide)-Based Green Flocculant. Iran. Polym. J. 2016, 25, 349–362. [Google Scholar] [CrossRef]
- Huang, X.; Gao, B.; Rong, H.; Yue, Q.; Zhang, Y.; Teng, P. Effect of Using Polydimethyldiallylammonium Chloride as Coagulation Aid on Polytitanium Salt Coagulation Performance, Floc Properties and Sludge Reuse. Sep. Purif. Technol. 2015, 143, 64–71. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, X.; Nie, F. Flocculation Performance and Microstructural Morphology of PFM-PDMDAAC for Treatment of Raw Water with Low Temperature and Low Turbidity in Winter. China Water Wastewater 2016, 3, 61–65. [Google Scholar]
- Zhang, Y.; Zhao, X.; Zhu, L.; Su, L. Turbidity Removal of Winter Taihu Lake Raw Water with Low Temperature Using Composite Coagulants of Polyaluminum Chloride and Polydimethyldiallylammonium Chloride in Process of Pre-chlorination. J. Nanjing Univ. Sci. Technol. 2008, 4, 506–511. [Google Scholar]
- Ma, F.; Meng, L.; Pang, C.; Jin, C.; Yao, J. Pilot scale treatment of low turbidity water using compound bioflocculant and polymerized aluminium ferrum chloride. J. Harbin Inst. Technol. 2009, 03, 441–444. [Google Scholar]























| Water | Characteristic | Unit | Value |
|---|---|---|---|
| Simulated water | Temperature | °C | 5 |
| Turbidity | NTU | 10 | |
| pH | – | 7.8 ± 0.2 | |
| UV254 | mg/L | 10 | |
| TOC | mg/L | 4–8 | |
| Natural water (Xiangjiang River) | Temperature | °C | 7.5 ± 0.3 |
| Turbidity | NTU | 14–18 | |
| pH | – | 7.24 ± 0.2 |
| pH | The Dosage of PATC-PDMDAAC (mg/L) | EM (nm) | EX (nm) | Fluorescence Intensity |
|---|---|---|---|---|
| pH = 5.0 | 0 | 473 | 274 | 5558 |
| 0.45 | 443 | 274 | 3069 | |
| 0.90 | 461 | 272 | 2822 | |
| 1.35 | 434 | 270 | 2990 | |
| 1.80 | 446 | 270 | 2655 | |
| 2.25 | 446 | 270 | 2589 | |
| pH = 7.0 | 0 | 485 | 270 | 7219 |
| 0. 45 | 476 | 270 | 5504 | |
| 0.90 | 446 | 256 | 3199 | |
| 1.35 | 446 | 260 | 2802 | |
| 1.80 | 437 | 260 | 3079 | |
| 2.25 | 437 | 262 | 2843 | |
| pH = 9.0 | 0 | 476 | 272 | 7053 |
| 0. 45 | 452 | 274 | 5603 | |
| 0.90 | 440 | 258 | 3360 | |
| 1.35 | 464 | 266 | 2532 | |
| 1.80 | 455 | 262 | 2856 | |
| 2.25 | 443 | 262 | 2717 |
| Dosage | 1.35 mg/L | 1.80 mg/L | 2.25 mg/L | 2.70 mg/L | ||||
|---|---|---|---|---|---|---|---|---|
| KN0 (× 10−4 s) | R2 | KN0 (× 10−4 s) | R2 | KN0 (× 10−4 s) | R2 | KN0 (× 10−4 s) | R2 | |
| PATC-PDMDAAC | 21.4 | 0.833 | 27.8 | 0.995 | 23.4 | 0.858 | 24.6 | 0.909 |
| Coagulants | The Characteristics of Water | Dosage mg/L | Removal Efficiency | Reference | ||
|---|---|---|---|---|---|---|
| Initial Turbidity NTU | Temperature °C | pH | ||||
| PFM-PDMDAAC | 17.4–21.5 | 7–9 | 7.15–7.32 | 3 | 95% | [40] |
| PAC | 6 | 90% | ||||
| PFS | 6 | 92% | ||||
| PAC-PDMDAAC | 25–26 | 5–9 | / | 2.66–2.53 | Below2 NTU | [41] |
| PAC | 3.17–3.11 | |||||
| CBF+PAFC | 2.05 | 4.7 | 8.2 | 2 + 15 | 0.48 NTU | [42] |
| PAFC | 20 | 0.8 NTU | ||||
| Material | Specification | Unit | Unit Price (RMB/t) | Consumption | Total (RMB/t) |
|---|---|---|---|---|---|
| AlCl3 | Industrial grade | t | 1550 | 0.0254 | 39 |
| Na2SiO3 | Industrial grade | t | 2880 | 0.0159 | 46 |
| TiCl4 | Industrial grade | t | 7000 | 0.0057 | 40 |
| NaOH | Industrial grade | t | 2600 | 0.0011 | 3 |
| H2SO4 | Industrial grade | t | 850 | 0.0322 | 27 |
| PDMDAAC | Industrial grade | t | 8500 | 0.0036 | 30 |
| PATC-PDMDAAC | – | – | – | – | 185 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Liao, L.; Zhu, G. Performance of PATC-PDMDAAC Composite Coagulants in Low-Temperature and Low-Turbidity Water Treatment. Materials 2019, 12, 2824. https://doi.org/10.3390/ma12172824
Zhang P, Liao L, Zhu G. Performance of PATC-PDMDAAC Composite Coagulants in Low-Temperature and Low-Turbidity Water Treatment. Materials. 2019; 12(17):2824. https://doi.org/10.3390/ma12172824
Chicago/Turabian StyleZhang, Peng, Lina Liao, and Guocheng Zhu. 2019. "Performance of PATC-PDMDAAC Composite Coagulants in Low-Temperature and Low-Turbidity Water Treatment" Materials 12, no. 17: 2824. https://doi.org/10.3390/ma12172824
APA StyleZhang, P., Liao, L., & Zhu, G. (2019). Performance of PATC-PDMDAAC Composite Coagulants in Low-Temperature and Low-Turbidity Water Treatment. Materials, 12(17), 2824. https://doi.org/10.3390/ma12172824