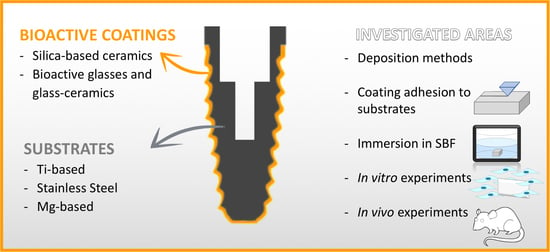
Bioactive Glass and Silicate-Based Ceramic Coatings on Metallic Implants: Open Challenge or Outdated Topic?
Abstract
:1. Introduction
2. Microstructural Features of Metallic Substrates
2.1. Ti and Ti Alloys
2.2. Stainless Steel
2.3. Mg and Mg Alloys
3. Microstructure, Physical Features and Applications of Bioactive Glasses and Silica-Based Bioceramics
3.1. Bioactive Glasses
3.2. Silica-Based Ceramics
4. Deposition Methods and Physical Properties of the Coatings
5. Coating-Substrate Adhesion Strength
6. Experiments in SBF Solution
7. In Vitro Experiments
7.1. In Vitro Behaviour of Ti-Based Substrates Coated by Bioactive Silica-BASED ceramics
7.2. In Vitro Behaviour of Bioactive Glass Coated Ti-Based Substrates
7.3. In Vitro Behaviour of Mg-Based Substrates Coated by Bioactive Silica-Based Ceramics
7.4. Antibacterial Properties of Bioactive Coatings
8. In Vivo Experiments
8.1. In Vivo Evaluation of Bioactive Coatings on Ti-Based Implants
8.2. In Vivo Evaluation of Bioactive Coatings on Stainless Steel Implants
8.3. In Vivo Evaluation of Bioactive Coatings on Mg-Based Implants
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Catauro, M.; Papale, F.; Bollino, F. Coatings of titanium substrates with xCaO (1 − x)SiO2 sol-gel materials: Characterization, bioactivity and biocompatibility evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Moritz, N.; Rossi, S.; Vedel, E.; Tirri, T.; Ylänen, H.; Aro, H.; Närhi, T. Implants coated with bioactive glass by CO2-laser, an in vivo study. J. Mater. Sci. Mater. Med. 2004, 15, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Beloti, M.M.; Rollo, J.M.; Itman Filho, A.; Rosa, A.L. In vitro biocompatibility of duplex stainless steel with and without 0.2% niobium. J. Appl. Biomater. Biomech. 2004, 2, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef] [Green Version]
- Talha, M.; Behera, C.K.; Sinha, O.P. A review on nickel-free nitrogen containing austenitic stainless steels for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3563–3575. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Palmquist, A.; Omar, O.M.; Esposito, M.; Lausmaa, J.; Thomsen, P. Titanium oral implants: Surface characteristics, interface biology and clinical outcome. J. R. Soc. Interface 2010, 7, S515–S527. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, X.Y.; Feng, Y.F.; Ma, Z.S.; Wang, J.; Ma, T.C.; Qi, W.; Lei, W.; Wang, L. Osseointegration of chitosan coated porous titanium alloy implant by reactive oxygen species-mediated activation of the PI3K/AKT pathway under diabetic conditions. Biomaterials 2015, 36, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Lin, D.J.; Hung, F.Y.; Jakfar, S.; Yeh, M.L. Tailored coating chemistry and interfacial properties for construction of bioactive ceramic coatings on magnesium biomaterial. Mater. Des. 2016, 89, 235–244. [Google Scholar] [CrossRef]
- Lee, H.P.; Lin, D.J.; Yeh, M.L. Phenolic modified ceramic coating on biodegradable mg alloy: The improved corrosion resistance and osteoblast-like cell activity. Materials 2017, 10, 696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cai, S.; Xu, G.; Shen, S.; Li, Y.; Zhang, M.; Wu, X. Corrosion behavior of mesoporous bioglass-ceramic coated magnesium alloy under applied forces. J. Mech. Behav. Biomed. Mater. 2016, 56, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Zhu, R.F.; Lu, Y.P.; Xiao, G.Y.; He, K.; Yuan, Y.F.; Ma, X.N.; Li, Y. Effect of sandblasting intensity on microstructures and properties of pure titanium microarc oxidation coatings in an optimized composite technique. Appl. Surf. Sci. 2014, 292, 204–212. [Google Scholar] [CrossRef]
- Civantos, A.; Martínez-Campos, E.; Ramos, V.; Elvira, C.; Gallardo, A.; Abarrategi, A. Titanium coatings and surface modifications: Toward clinically useful bioactive implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F. Biocompatibility improvement of titanium implants by coating with hybrid materials synthesized by sol-gel technique. J. Biomed. Mater. Res. A 2014, 102, 4473–4479. [Google Scholar] [CrossRef]
- Midha, S.; Kim, T.B.; van den Bergh, W.; Lee, P.D.; Jones, J.R.; Mitchell, C.A. Preconditioned 70S30C bioactive glass foams promote osteogenesis in vivo. Acta Biomater. 2013, 9, 9169–9182. [Google Scholar] [CrossRef]
- Niinomi, M. Recent metallic materials for biomedical applications. Met. Mater. Trans. A 2002, 33, 477–486. [Google Scholar] [CrossRef]
- Deen, J.T.; Clay, T.B.; Iams, D.A.; Horodyski, M.; Parvataneni, H.K. Proximal tibial resorption in a modern total knee prosthesis. Arthroplast. Today 2017, 4, 244–248. [Google Scholar] [CrossRef] [Green Version]
- Inoue, K.; Suenaga, N.; Oizumi, N.; Yamaguchi, H.; Miyoshi, N.; Taniguchi, N.; Munemoto, M.; Egawa, T.; Tanaka, Y. Humeral bone resorption after anatomic shoulder arthroplasty using an uncemented stem. J. Shoulder Elbow Surg. 2017, 26, 1984–1989. [Google Scholar] [CrossRef]
- Spormann, C.; Durchholz, H.; Audigé, L.; Flury, M.; Schwyzer, H.K.; Simmen, B.R.; Kolling, C. Patterns of proximal humeral bone resorption after total shoulder arthroplasty with an uncemented rectangular stem. J. Shoulder Elbow Surg. 2014, 23, 1028–1035. [Google Scholar] [CrossRef]
- Cilla, M.; Checa, S.; Duda, G.N. Strain shielding inspired re-design of proximal femoral stems for total hip arthroplasty. J. Orthop. Res. 2017, 35, 2534–2544. [Google Scholar] [CrossRef] [PubMed]
- Bayata, F.; Yildiz, C. The mechanical behaviors of various dental implant materials under fatigue. Adv. Mater. Sci. Eng. 2018, 2018, 5047319. [Google Scholar] [CrossRef]
- Cui, W.; Liu, Y. Fatigue behavior of Ti50Zr alloy for dental applications. J. Alloys Compd. 2019, 793, 212–219. [Google Scholar] [CrossRef]
- Najdahmadi, A.; Zarei-Hanzaki, A.; Farghadani, E. Mechanical properties enhancement in Ti–29Nb–13Ta–4.6Zr alloy via heat treatment with no detrimental effect on its biocompatibility. Mater. Des. 2014, 54, 786–791. [Google Scholar] [CrossRef]
- Herrera, A.; Mateo, J.; Lobo-Escolar, A.; Panisello, J.J.; Ibarz, E.; Gracia, L. Long-term outcomes of a new model of anatomical hydroxyapatite-coated hip prosthesis. J. Arthroplast. 2013, 28, 1160–1166. [Google Scholar] [CrossRef] [PubMed]
- Dallago, M.; Fontanari, V.; Torresani, E.; Leoni, M.; Pederzolli, C.; Potrich, C.; Benedetti, M. Fatigue and biological properties of Ti-6Al-4V ELI cellular structures with variously arranged cubic cells made by selective laser melting. J. Mech. Behav. Biomed. Mater. 2018, 78, 381–394. [Google Scholar] [CrossRef]
- Vladescu, A.; Mihai Cotrut, C.; Ak Azem, F.; Bramowicz, M.; Pana, I.; Braic, V.; Birlik, I.; Kiss, A.; Braic, M.; Abdulgader, R.; et al. Sputtered Si and Mg doped hydroxyapatite for biomedical applications. Biomed. Mater. 2018, 13, 025011. [Google Scholar] [CrossRef] [Green Version]
- Hedia, H.S.; Fouda, N. Design optimization of cementless hip prosthesis coating through functionally graded material. Comput. Mater. Sci. 2014, 87, 83–87. [Google Scholar] [CrossRef]
- Wiskott, H.W.; Belser, U.C. Lack of integration of smooth titanium surfaces: A working hypothesis based on strains generated in the surrounding bone. Clin. Oral Implant. Res. 1999, 10, 429–444. [Google Scholar] [CrossRef]
- Korabi, R.; Shemtov-Yona, K.; Rittel, D. On stress/strain shielding and the material stiffness paradigm for dental implants. Clin. Implant. Dent. Relat. Res. 2017, 19, 935–943. [Google Scholar] [CrossRef]
- Nagano, M.; Nakamura, T.; Kokubo, T.; Tanahashi, M.; Ogawa, M. Differences of bone bonding ability and degradation behaviour in vivo between amorphous calcium phosphate and highly crystalline hydroxyapatite coating. Biomaterials 1996, 17, 1771–1777. [Google Scholar] [CrossRef]
- Lin, D.Y.; Wang, X.X. A novel method to synthesize hydroxyapatite coating with hierarchical structure. Colloids Surf. B Biointerfaces 2011, 82, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Bandyopadhyay, A.; Bose, S. Induction plasma sprayed nano hydroxyapatite coatings on titanium for orthopaedic and dental implants. Surf. Coat. Technol. 2011, 205, 2785–2792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsui, Y.C.; Doyle, C.; Clyne, T.W. Plasma sprayed hydroxyapatite coatings on titanium substrates. Part 1: Mechanical properties and residual stress levels. Biomaterials 1998, 19, 2015–2029. [Google Scholar] [CrossRef]
- Kweh, S.W.; Khor, K.A.; Cheang, P. An in vitro investigation of plasma sprayed hydroxyapatite (HA) coatings produced with flame-spheroidized feedstock. Biomaterials 2002, 23, 775–785. [Google Scholar] [CrossRef]
- Bauer, T.W.; Geesink, R.C.; Zimmerman, R.; McMahon, J.T. Hydroxyapatite-coated femoral stems. Histological analysis of components retrieved at autopsy. J. Bone Joint Surg. Am. 1991, 73, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, H.; Brunello, G.; Gardin, C.; Ferroni, L.; Badocco, D.; Pastore, P.; Sivolella, S.; Zavan, B.; Biasetto, L. Bioactive sphene-based ceramic coatings on cpTi substrates for dental implants: An in vitro study. Materials 2018, 11, 2234. [Google Scholar] [CrossRef]
- Bloyer, D.R.; Gomez-Vega, J.M.; Saiz, E.; McNaney, J.M.; Cannon, R.M.; Tomsia, A.P. Fabrication and characterization of a bioactive glass coating on titanium implant alloys. Acta Mater. 1999, 47, 4221–4224. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Zhu, Q.; Qi, H.; Liu, X.; Ma, M.; Chen, Q. A facile flow-casting production of bioactive glass coatings on porous titanium for bone tissue engineering. Materials 2018, 11, 1540. [Google Scholar] [CrossRef]
- Fu, Y.C.; Chen, C.H.; Wang, C.Z.; Wang, Y.H.; Chang, J.K.; Wang, G.J.; Ho, M.L.; Wang, C.K. Preparation of porous bioceramics using reverse thermo-responsive hydrogels in combination with rhBMP-2 carriers: In vitro and in vivo evaluation. J. Mech. Behav. Biomed. Mater. 2013, 27, 64–76. [Google Scholar] [CrossRef] [Green Version]
- Hankenson, K.D.; Gagne, K.; Shaughnessy, M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Adv. Drug. Deliv. Rev. 2015, 94, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kose, N.; Çaylak, R.; Pekşen, C.; Kiremitçi, A.; Burukoglu, D.; Koparal, S.; Doğan, A. Silver ion doped ceramic nano-powder coated nails prevent infection in open fractures: In vivo study. Injury 2016, 47, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Sivolella, S.; Stellini, E.; Brunello, G.; Gardin, C.; Ferroni, L.; Bressan, E.; Zavan, B. Silver nanoparticles in alveolar bone surgery devices. J. Nanomater. 2012, 2012, 975842. [Google Scholar] [CrossRef]
- Jäger, M.; Jennissen, H.P.; Dittrich, F.; Fischer, A.; Köhling, H.L. Antimicrobial and osseointegration properties of nanostructured titanium orthopaedic implants. Materials 2017, 10, 1302. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, G.; Francetti, L.; Barbaro, B.; Del Fabbro, M. Novel surfaces and osseointegration in implant dentistry. J. Investig. Clin. Dent. 2018, 9, e12349. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Trobos, M.; Thomsen, P.; Palmquist, A. Commercially pure titanium (cp-Ti) versus titanium alloy (Ti6Al4V) materials as bone anchored implants—Is one truly better than the other? Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, M.Y.; Sulong, A.B.; Muhamad, N.; Raza, M.R.; Ramli, M.I. Incorporation of wollastonite bioactive ceramic with titanium for medical applications: An overview. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 884–895. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.C.; Tokuhara, C.K.; Rocha, L.A.; Oliveira, R.C.; Lisboa-Filho, P.N.; Costa Pessoa, J. Vanadium ionic species from degradation of Ti-6Al-4V metallic implants: In vitro cytotoxicity and speciation evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 96, 730–739. [Google Scholar] [CrossRef]
- Xu, W.; Li, M.; Wen, C.; Lv, S.; Liu, C.; Lu, X.; Qu, X. The mechanical properties and in vitro biocompatibility of PM-fabricated Ti-28Nb-35.4Zr alloy for orthopedic implant applications. Materials 2018, 11, 531. [Google Scholar] [CrossRef]
- Okazaki, Y.; Gotoh, E. Comparison of metal release from various metallic biomaterials in vitro. Biomaterials 2005, 26, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Atapour, M.; Pilchak, A.L.; Frankel, G.S.; Williams, J.C. Corrosion behavior of beta titanium alloys for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2011, 31, 885–891. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, Y.; Xia, L.; Zhao, C.; Chen, L.; Yi, D.; Chang, J.; Huang, L.; Zheng, X.; Zhu, H.; et al. Fabrication of nano-structured calcium silicate coatings with enhanced stability, bioactivity and osteogenic and angiogenic activity. Colloids Surf. B Biointerfaces 2015, 126, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, P.; Cirera, A.; Dotor, J.; Gil, F.J.; Galindo-Moreno, P.; Aparicio, C. In vitro cell response on CP-Ti surfaces functionalized with TGF-β1 inhibitory peptides. J. Mater. Sci. Mater. Med. 2018, 29, 73. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.H.; Yoon, S.J.; Lee, D.W. Preparation and evaluation of dexamethasone (DEX)/Growth and Differentiation Factor-5 (GDF-5) surface-modified titanium using β-cyclodextrin-conjugated heparin (CD-Hep) for enhanced osteogenic activity in vitro and in vivo. Int. J. Mol. Sci. 2017, 18, 1695. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Jia, Z.; Xiong, P.; Yan, J.; Li, Y.; Li, M.; Cheng, Y.; Zheng, Y. Bioinspired and biomimetic AgNPs/gentamicin-embedded silk fibroin coatings for robust antibacterial and osteogenetic applications. ACS Appl. Mater. Interfaces 2017, 9, 25830–25846. [Google Scholar] [CrossRef]
- Omar, S.; Repp, F.; Desimone, P.M.; Weinkamer, R.; Wagermaier, W.; Ceré, S.; Ballarre, J. Sol–gel hybrid coatings with strontium-doped 45S5 glass particles for enhancing the performance of stainless steel implants: Electrochemical, bioactive and in vivo response. J. Non-Cryst. Solids 2015, 425, 1–10. [Google Scholar] [CrossRef]
- Sedriks, A.J. Corrosion of Stainless Steels; Wiley: New York, NY, USA, 1996. [Google Scholar]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic biomaterials: Current challenges and opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Sumita, M.; Hanawa, T.; Teoh, S.H. Development of nitrogen-containing nickel-free austenitic stainless steels for metallic biomaterials-review. Mater. Sci. Eng. C Mater. Biol. Appl. 2004, 24, 753–760. [Google Scholar] [CrossRef]
- Thomann, U.I.; Uggowitzer, P.J. Wear–corrosion behavior of biocompatible austenitic stainless steels. Wear 2000, 239, 48–58. [Google Scholar] [CrossRef]
- Ballarre, J.; Liu, Y.; Mendoza, E.; Schell, H.; Díaz, F.; Orellano, J.C.; Fratzl, P.; García, C.; Ceré, S.M. Enhancing low cost stainless steel implants: Bioactive silica-based sol-gel coatings with wollastonite particles. Int. J. Nano Biomater. 2012, 4, 33–53. [Google Scholar] [CrossRef]
- Ballarre, J.; López, D.A.; Cavalieri, A.L. Frictional and adhesive behavior of organic–inorganic hybrid coatings on surgical grade stainless steel using nano-scratching technique. Wear 2009, 266, 1165–1170. [Google Scholar] [CrossRef]
- Farraro, K.F.; Kim, K.E.; Woo, S.L.; Flowers, J.R.; McCullough, M.B. Revolutionizing orthopaedic biomaterials: The potential of biodegradable and bioresorbable magnesium-based materials for functional tissue engineering. J. Biomech. 2014, 47, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Fathi, M.; Savabi, O.; Razavi, S.M.; Heidari, F.; Manshaei, M.; Vashaee, D.; Tayebi, L. In vivo study of nanostructured diopside (CaMgSi2O6) coating on magnesium alloy as biodegradable orthopedic implants. Appl. Surf. Sci. 2014, 313, 60–66. [Google Scholar] [CrossRef]
- Razavi, M.; Fathi, M.; Savabi, O.; Tayebi, L.; Vashaee, D. Improvement of in vitro behavior of an Mg alloy using a nanostructured composite bioceramic coating. J. Mater. Sci. Mater. Med. 2018, 29, 159. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Fathi, M.; Savabi, O.; Vashaee, D.; Tayebi, L. In vivo biocompatibility of Mg implants surface modified by nanostructured merwinite/PEO. J. Mater. Sci. Mater. Med. 2015, 26, 184. [Google Scholar] [CrossRef] [PubMed]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Rau, J.V.; Antoniac, I.; Fosca, M.; De Bonis, A.; Blajan, A.I.; Cotrut, C.; Graziani, V.; Curcio, M.; Cricenti, A.; Niculescu, M.; et al. Glass-ceramic coated Mg-Ca alloys for biomedical implant applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 64, 362–369. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wen, C.; Hodgson, P.; Li, Y. Effects of alloying elements on the corrosion behavior and biocompatibility of biodegradable magnesium alloys: A review. J. Mater. Chem. B 2014, 2, 1912–1933. [Google Scholar] [CrossRef]
- Li, L.; Gao, J.; Wang, Y. Evaluation of cyto-toxicity and corrosion behavior of alkali-heat-treated magnesium in simulated body fluid. Surf. Coat. Technol. 2004, 185, 92–98. [Google Scholar] [CrossRef]
- Li, X.; Liu, X.; Wu, S.; Yeung, K.W.K.; Zheng, Y.; Chu, P.K. Design of magnesium alloys with controllable degradation for biomedical implants: From bulk to surface. Acta Biomater. 2016, 45, 2–30. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Curtin, J.; Duffy, B.; Jaiswal, S. Biodegradable magnesium alloys for orthopaedic applications: A review on corrosion, biocompatibility and surface modifications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 948–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Hench, L.L. Bioactive materials. Ceram. Int. 1996, 22, 493–507. [Google Scholar] [CrossRef]
- Hench, L.L.; Paschall, H.A. Direct chemical bond of bioactive glass-ceramic materials to bone and muscle. J. Biomed. Mater. Res. 1973, 7, 25–42. [Google Scholar] [CrossRef] [PubMed]
- Xynos, I.D.; Hukkanen, M.V.; Batten, J.J.; Buttery, L.D.; Hench, L.L.; Polak, J.M. Bioglass 45S5 stimulates osteoblast turnover and enhances bone formation In vitro: Implications and applications for bone tissue engineering. Calcif. Tissue Int. 2000, 67, 321–329. [Google Scholar] [CrossRef]
- Stanley, H.R.; Hall, M.B.; Clark, A.E.; King, C.J., III; Hench, L.L.; Berte, J.J. Using 45S5 bioglass cones as endosseous ridge maintenance implants to prevent alveolar ridge resorption: A 5-year evaluation. Int. J. Oral Maxillofac. Implant. 1997, 12, 95–105. [Google Scholar]
- Rust, K.R.; Singleton, G.T.; Wilson, J.; Antonelli, P.J. Bioglass middle ear prosthesis: Long-term results. Am. J. Otol. 1996, 17, 371–374. [Google Scholar]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Du Min, Q.; Bian, Z.; Jiang, H.; Greenspan, D.C.; Burwell, A.K.; Zhong, J.; Tai, B.J. Clinical evaluation of a dentifrice containing calcium sodium phosphosilicate (novamin) for the treatment of dentin hypersensitivity. Am. J. Dent. 2008, 21, 210–214. [Google Scholar] [PubMed]
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, H.; Rath, C.; Kumar, A.S.; Manna, P.P.; Singh, S.P. Structural, physico-mechanical and in-vitro bioactivity studies on SiO2-CaO-P2O5-SrO-Al2O3 bioactive glasses. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, D.; Bianchi, M.; Graziani, G.; Gambardella, A.; Berni, M.; Russo, A.; Cannillo, V. Pulsed Electron Deposition of nanostructured bioactive glass coatings for biomedical applications. Ceram. Int. 2017, 43, 15862–15867. [Google Scholar] [CrossRef]
- Drouet, C.; Leriche, A.; Hampshire, S.; Kashani, M.; Stamboulis, A.; Iafisco, M.; Tampieri, A. Types of ceramics: Material class. In Advances in Ceramic Biomaterials (Materials, Devices and Challenges); Palmero, P., Cambier, F., Barra, E.D., Eds.; Woodhead Publishing: Sawston, Cambridge, UK, 2017; pp. 21–82. [Google Scholar] [CrossRef]
- Kinnunen, I.; Aitasalo, K.; Pöllönen, M.; Varpula, M. Reconstruction of orbital floor fractures using bioactive glass. J. Craniomaxillofac. Surg. 2000, 28, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Van Gestel, N.A.; Geurts, J.; Hulsen, D.J.; van Rietbergen, B.; Hofmann, S.; Arts, J.J. Clinical applications of S53P4 Bioactive glass in bone healing and osteomyelitic treatment: A literature review. Biomed. Res. Int. 2015, 2015, 684826. [Google Scholar] [CrossRef]
- Tanner, M.C.; Heller, R.; Westhauser, F.; Miska, M.; Ferbert, T.; Fischer, C.; Gantz, S.; Schmidmaier, G.; Haubruck, P. Evaluation of the clinical effectiveness of bioactive glass (S53P4) in the treatment of non-unions of the tibia and femur: Study protocol of a randomized controlled non-inferiority trial. Trials 2018, 19, 299. [Google Scholar] [CrossRef]
- Peltola, M.J.; Aitasalo, K.M.; Suonpää, J.T.; Yli-Urpo, A.; Laippala, P.J.; Forsback, A.P. Frontal sinus and skull bone defect obliteration with three synthetic bioactive materials. A comparative study. J. Biomed. Mater. Res. B Appl. Biomater. 2003, 66, 364–372. [Google Scholar] [CrossRef]
- Maçon, A.L.; Kim, T.B.; Valliant, E.M.; Goetschius, K.; Brow, R.K.; Day, D.E.; Hoppe, A.; Boccaccini, A.R.; Kim, I.Y.; Ohtsuki, C.; et al. A unified in vitro evaluation for apatite-forming ability of bioactive glasses and their variants. J. Mater. Sci. Mater. Med. 2015, 26, 115. [Google Scholar] [CrossRef]
- Montazerian, M.; Zanotto, E.D. History and trends of bioactive glass-ceramics. J. Biomed. Mater. Res. A 2016, 104, 1231–1249. [Google Scholar] [CrossRef] [PubMed]
- Peitl, O.; LaTorre, G.P.; Hench, L.L. Effect of crystallization on apatite layer formation of bioactive glass 45S5. J. Biomed. Mater. Res. 1996, 30, 509–514. [Google Scholar] [CrossRef]
- Peitl, O.; Zanotto, E.D.; Hench, L.L. Highly bioactive P2O5–Na2O–CaO–SiO2 glass-ceramics. J. Non-Cryst. Solids 2001, 292, 115–126. [Google Scholar] [CrossRef]
- Thompson, I.D.; Hench, L.L. Mechanical properties of bioactive glasses, glass-ceramics and composites. Proc. Inst. Mech. Eng. H 1998, 212, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Bellucci, D.; Cannillo, V.; Sola, A. Coefficient of thermal expansion of bioactive glasses: Available literature data and analytical equation estimates. Ceram. Int. 2011, 37, 2963–2972. [Google Scholar] [CrossRef]
- Cattini, A.; Łatka, L.; Bellucci, D.; Bolelli, G.; Sola, A.; Lusvarghi, L.; Pawłowski, L.; Cannillo, V. Suspension plasma sprayed bioactive glass coatings: Effects of processing on microstructure, mechanical properties and in-vitro behavior. Surf. Coat. Technol. 2013, 220, 52–59. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Brauer, D.S.; Hupa, L. Bioactive Glasses: Fundamentals, Technology and Applications; Royal Society of Chemistry: London, UK, 2016. [Google Scholar] [CrossRef]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable Materials and Metallic Implants—A Review. J. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chang, J. A review of bioactive silicate ceramics. Biomed. Mater. 2013, 8, 032001. [Google Scholar] [CrossRef]
- Juraski, A.C.; Rodas, A.C.D.; Elsayed, H.; Bernardo, E.; Soares, V.O.; Daguano, J. The in vitro bioactivity, degradation, and cytotoxicity of polymer-derived wollastonite-diopside glass-ceramics. Materials 2017, 10, 425. [Google Scholar] [CrossRef]
- Elsayed, H.; Sinico, M.; Secco, M.; Zorzi, F.; Colombo, P.; Bernardo, E. B-doped hardystonite bioceramics from preceramic polymers and fillers: Synthesis and application to foams and 3D-printed scaffolds. J. Eur. Ceram. Soc. 2017, 37, 1757–1767. [Google Scholar] [CrossRef]
- Götz, W.; Tobiasch, E.; Witzleben, S.; Schulze, M. Effects of silicon compounds on biomineralization, osteogenesis, and hard tissue formation. Pharmaceutics 2019, 11, 117. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Sepantafar, M.; Ostadrahimi, A. The role of bioinorganics in improving the mechanical properties of silicate ceramics as bone regenerative materials. J. Ceram. Sci. Tech. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Zocca, A.; Elsayed, H.; Bernardo, E.; Gomes, C.M.; Lopez-Heredia, M.A.; Knabe, C.; Colombo, P.; Günster, J. 3D-printed silicate porous bioceramics using a non-sacrificial preceramic polymer binder. Biofabrication 2015, 7, 025008. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, H.; Zocca, A.; Bernardo, E.; Gomes, C.M.; Günster, J.; Colombo, P. Development of bioactive silicate-based glass-ceramics from preceramic polymer and fillers. J. Eur. Ceram. Soc. 2015, 35, 731–739. [Google Scholar] [CrossRef]
- Heimann, R.B.; Lehmann, H.D. Bioceramic Coatings for Medical Implants: Trends and Techniques; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Long, L.H.; Chen, L.D.; Bai, S.Q.; Chang, J.; Lin, K.L. Preparation of dense β-CaSiO3 ceramic with high mechanical strength and HAp formation ability in simulated body fluid. J. Eur. Ceram. Soc. 2006, 26, 1701–1706. [Google Scholar] [CrossRef]
- Gou, Z.R.; Chang, J.; Zhai, W.Y. Preparation and characterization of novel bioactive dicalcium silicate ceramics. J. Eur. Ceram. Soc. 2005, 25, 1507–1514. [Google Scholar] [CrossRef]
- Heimann, R.B. Important Ceramic Phase Systems. In Classic and Advanced Ceramics: From Fundamentals to Applications; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 55–97. [Google Scholar] [CrossRef]
- Zhao, W.; Chang, J. Preparation and characterization of novel tricalcium silicate bioceramics. J. Biomed. Mater. Res. A 2005, 73, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Thieme, C.; Rüssel, C. Thermal expansion behavior of SrSiO3 and Sr2SiO4 determined by high-temperature X-ray diffraction and dilatometry. J. Mater. Sci. 2015, 50, 5533–5539. [Google Scholar] [CrossRef]
- Bullard, J.W. A determination of hydration mechanisms for tricalcium silicate using a kinetic cellular automaton model. J. Am. Ceram. Soc. 2008, 91, 2088–2097. [Google Scholar] [CrossRef]
- Tan, Y.M.; Tan, C.Y.; Ramesh, S.; The, Y.C.; Ching, Y.C.; Lwin, N.; Yap, B.K.; Agrawal, D. Study on the effects of milling time and sintering temperature on the sinterability of forsterite (Mg2SiO4). J. Ceram. Soc. Jpn. 2015, 123, 1032–1037. [Google Scholar] [CrossRef]
- McColm, I.J. Dictionary of Ceramic Science and Engineering; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Wu, C.; Chang, J. A novel akermanite bioceramic: Preparation and characteristics. J. Biomater. Appl. 2006, 21, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Haussühl, S.; Liebertz, J. Elastic and thermoelastic properties of synthetic Ca2MgSi2O7 (åkermanite) and Ca2ZnSi2O7 (hardystonite). Phys. Chem. Miner. 2004, 31, 565–567. [Google Scholar] [CrossRef]
- Chen, X.; Ou, J.; Wei, Y.; Huang, Z.; Kang, Y.; Yin, G. Effect of MgO contents on the mechanical properties and biological performances of bioceramics in the MgO-CaO-SiO2 system. J. Mater. Sci. Mater. Med. 2010, 21, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chang, J.; Wang, J.; Ni, S.; Zhai, W. Preparation and characteristics of a calcium magnesium silicate (bredigite) bioactive ceramic. Biomaterials 2005, 26, 2925–2931. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chang, J. Synthesis and in vitro bioactivity of bredigite powders. J. Biomater. Appl. 2007, 21, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Nonami, T.; Tsutsumi, S. Study of diopside ceramics for biomaterials. J. Mater. Sci. Mater. Med. 1999, 10, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Liu, X.; Zheng, X.; Ding, C. Plasma-sprayed diopside coatings for biomedical applications. Surf. Coat. Tech. 2004, 185, 340–345. [Google Scholar] [CrossRef]
- Hafezi-Ardakani, M.; Moztarzadeh, F.; Rabiee, M.; Talebi, A.R.; Abasi-shahni, M.; Fesahat, F.; Sadeghian, F. Sol-gel synthesis and apatite-formation ability of nanostructure merwinite (Ca3MgSi2O8) as a novel bioceramic. J. Ceram. Process. Res. 2010, 11, 765–768. [Google Scholar]
- Ou, J.; Kang, Y.; Huang, Z.; Chen, X.; Wu, J.; Xiao, R.; Yin, G. Preparation and in vitro bioactivity of novel merwinite ceramic. Biomed. Mater. 2008, 3, 015015. [Google Scholar] [CrossRef]
- Pantić, J.; Urbanovich, V.; Poharc-Logar, V.; Jokić, B.; Stojmenović, M.; Kremenović, A.; Matović, B. Synthesis and characterization of high-pressure and high-temperature sphene (CaTiSiO5). Phys. Chem. Miner. 2014, 41, 775–782. [Google Scholar] [CrossRef]
- Wu, C.; Ramaswamy, Y.; Liu, X.; Wang, G.; Zreiqat, H. Plasma-sprayed CaTiSiO5 ceramic coating on Ti-6Al-4V with excellent bonding strength, stability and cellular bioactivity. J. R. Soc. Interface 2009, 6, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.C.; Volkmann, E.; Yilmaz, R.; Wolf, A.; Treccani, L.; Rezwan, K. Mechanical evaluation of calcium-zirconium-silicate (baghdadite) obtained by a direct solid-state synthesis route. J. Mech. Behav. Biomed. Mater. 2014, 34, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Al-Hermezi, H.M.; McKie, D.; Hall, A.J. Baghdadite, a new calcium zirconium silicate mineral from Iraq. Mineral. Mag. 1986, 50, 119–123. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. On implant surfaces: A review of current knowledge and opinions. Int. J. Oral Maxillofac. Implant. 2010, 25, 63–74. [Google Scholar]
- Lynn, A.K.; DuQuesnay, D.L. Hydroxyapatite-coated Ti-6Al-4V part 1: The effect of coating thickness on mechanical fatigue behaviour. Biomaterials 2002, 23, 1937–1946. [Google Scholar] [CrossRef]
- Biasetto, L.; Elsayed, H.; Bonollo, F.; Colombo, P. Polymer-derived sphene biocoating on cp-Ti substrates for orthopedic and dental implants. Surf. Coat. Technol. 2016, 301, 140–147. [Google Scholar] [CrossRef]
- Biasetto, L.; Elsayed, H. Sphene silicate ceramic coatings on cpTi substrates: Process upgrade. Surf. Coat. Technol. 2017, 321, 416–424. [Google Scholar] [CrossRef]
- Biasetto, L.; Bertolini, R.; Elsayed, H.; Ghiotti, A.; Bruschi, S. Use of cryogenic machining to improve the adhesion of sphene bioceramic coatings on titanium substrates for dental and orthopaedic applications. Ceram. Int. 2019, 45, 5941–5951. [Google Scholar] [CrossRef]
- Wang, G.; Lu, Z.; Liu, X.; Zhou, X.; Ding, C.; Zreiqat, H. Nanostructured glass-ceramic coatings for orthopaedic applications. J. R. Soc. Interface 2011, 8, 1192–1203. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wei, D.; Zhou, Y. Formation and structure of sphene/titania composite coatings on titanium formed by a hybrid technique of microarc oxidation and heat-treatment. Appl. Surf. Sci. 2011, 257, 3404–3411. [Google Scholar] [CrossRef]
- Ramaswamy, Y.; Wu, C.; Dunstan, C.R.; Hewson, B.; Eindorf, T.; Anderson, G.I.; Zreiqat, H. Sphene ceramics for orthopedic coating applications: An in vitro and in vivo study. Acta Biomater. 2009, 5, 3192–3204. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ramaswamy, Y.; Gale, D.; Yang, W.; Xiao, K.; Zhang, L.; Yin, Y.; Zreiqat, H. Novel sphene coatings on Ti-6Al-4V for orthopedic implants using sol-gel method. Acta Biomater. 2008, 4, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yu, J.; Xie, Y.; Huang, L.; Ye, X.; Zheng, X. Chemical stability and antimicrobial activity of plasma sprayed bioactive Ca2ZnSi2O7 coating. J. Mater. Sci. Mater. Med. 2011, 22, 2781–2789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, G.; Liu, Y.; Zhao, X.; Zou, D.; Zhu, C.; Jin, Y.; Huang, Q.; Sun, J.; Liu, X.; et al. The synergistic effect of hierarchical micro/nano-topography and bioactive ions for enhanced osseointegration. Biomaterials 2013, 34, 3184–3195. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Wu, C.; Ma, X.; Ji, H.; Zheng, X.; Chang, J. Preparation and in vitro evaluation of plasma-sprayed bioactive akermanite coatings. Biomed. Mater. 2012, 7, 065004. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xie, Y.; Ji, H.; Huang, L.; Zheng, X. Excellent stability of plasma-sprayed bioactive Ca3ZrSi2O9 ceramic coating on Ti–6Al–4V. Appl. Surf. Sci. 2010, 256, 4677–4681. [Google Scholar] [CrossRef]
- Yi, D.; Wu, C.; Ma, B.; Ji, H.; Zheng, X.; Chang, J. Bioactive bredigite coating with improved bonding strength, rapid apatite mineralization and excellent cytocompatibility. J. Biomater. Appl. 2014, 28, 1343–1353. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tao, S.; Ding, C. Bioactivity of plasma sprayed dicalcium silicate coatings. Biomaterials 2002, 23, 963–968. [Google Scholar] [CrossRef]
- Liu, X.Y.; Ding, C.X. Characterization of plasma sprayed wollastonite powder and coatings. Surf. Coat. Technol. 2002, 153, 173–177. [Google Scholar] [CrossRef]
- Garcia, E.; Miranzo, P.; Sainz, M.A. Thermally sprayed wollastonite and wollastonite-diopside compositions as new modulated bioactive coatings for metal implants. Ceram. Int. 2018, 44, 12896–12904. [Google Scholar] [CrossRef]
- Sanyal, S.; Shukla, M.; Dandapat, N.; Ghosh, S. In vitro evaluation of bioactive glass ceramic coating for application on Ti6Al4V based biomedical implants. J. Non-Cryst. Solids 2018, 500, 22–29. [Google Scholar] [CrossRef]
- Newman, S.D.; Lotfibakhshaiesh, N.; O’Donnell, M.; Walboomers, X.F.; Horwood, N.; Jansen, J.A.; Amis, A.A.; Cobb, J.P.; Stevens, M.M. Enhanced osseous implant fixation with strontium-substituted bioactive glass coating. Tissue Eng. Part A 2014, 20, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Bolelli, G.; Bellucci, D.; Cannillo, V.; Gadow, R.; Killinger, A.; Lusvarghi, L.; Müller, P.; Sola, A. Comparison between suspension plasma sprayed and high velocity suspension flame sprayed bioactive coatings. Surf. Coat. Technol. 2015, 280, 232–249. [Google Scholar] [CrossRef]
- Altomare, L.; Bellucci, D.; Bolelli, G.; Bonferroni, B.; Cannillo, V.; De Nardo, L.; Gadow, R.; Killinger, A.; Lusvarghi, L.; Sola, A.; et al. Microstructure and in vitro behaviour of 45S5 bioglass coatings deposited by high velocity suspension flame spraying (HVSFS). J. Mater. Sci. Mater. Med. 2011, 22, 1303–1319. [Google Scholar] [CrossRef] [PubMed]
- Van Oirschot, B.A.; Alghamdi, H.S.; Närhi, T.O.; Anil, S.; Al Farraj Aldosari, A.; van den Beucken, J.J.; Jansen, J.A. In vivo evaluation of bioactive glass-based coatings on dental implants in a dog implantation model. Clin. Oral Implant. Res. 2014, 25, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Van Oirschot, B.A.; Meijer, G.J.; Bronkhorst, E.M.; Närhi, T.; Jansen, J.A.; van den Beucken, J.J. Comparison of different surface modifications for titanium implants installed into the goat iliac crest. Clin. Oral Implant. Res. 2016, 27, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Mistry, S.; Roy, R.; Kundu, B.; Datta, S.; Kumar, M.; Chanda, A.; Kundu, D. Clinical outcome of hydroxyapatite coated, bioactive glass coated, and machined Ti6Al4V Threaded dental implant in human jaws: A short-term comparative study. Implant Dent. 2016, 25, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Bollino, F.; Papale, F.; Vecchio Ciprioti, S. Investigation on bioactivity, biocompatibility, thermal behavior and antibacterial properties of calcium silicate glass coatings containing Ag. J. Non-Cryst. Solids 2015, 422, 16–22. [Google Scholar] [CrossRef]
- Bagherpour, I.; Naghib, S.M.; Yaghtin, A.H. Synthesis and characterisation of nanostructured hardystonite coating on stainless steel for biomedical application. IET Nanobiotechnol. 2018, 12, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Bagherpour, I. Fabrication of hardystonite nano-bioceramic coating on 306L stainless steel substrate using electrophoretic method and evaluation of its corrosion resistance to improve medical performance. In The Minerals, Metals & Materials Series (eds) TMS 2019 148th Annual Meeting & Exhibition Supplemental Proceedings. The Minerals, Metals & Materials Series; Springer: Cham, Switzerland, 2019; pp. 143–154. [Google Scholar] [CrossRef]
- Ballarre, J.; Seltzer, R.; Mendoza, E.; Orellano, J.C.; Mai, Y.W.; García, C.; Ceré, S.M. Morphologic and nanomechanical characterization of bone tissue growth around bioactive sol–gel coatings containing wollastonite particles applied on stainless steel implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2011, 31, 545–552. [Google Scholar] [CrossRef]
- Yu, H.; Dong, Q.; Dou, J.; Pan, Y.; Chen, C. Structure and in vitro bioactivity of ceramic coatings on magnesium alloys by microarc oxidation. Appl. Surf. Sci. 2016, 388, 114–119. [Google Scholar] [CrossRef]
- Ye, X.; Cai, S.; Dou, Y.; Xu, G.; Huang, K.; Ren, M.; Wang, X. Bioactive glass–ceramic coating for enhancing the in vitro corrosion resistance of biodegradable Mg alloy. Appl. Surf. Sci. 2012, 259, 799–805. [Google Scholar] [CrossRef]
- Dou, Y.; Cai, S.; Ye, X.; Xu, G.; Huang, K.; Wang, X.; Ren, M. 45S5 bioactive glass–ceramic coated AZ31 magnesium alloy with improved corrosion resistance. Surf. Coat. Technol. 2013, 228, 154–161. [Google Scholar] [CrossRef]
- Niu, S.; Cai, S.; Liu, T.; Zhao, H.; Wang, X.; Ren, M.; Huang, K.; Wu, X. 45S5 bioactive glass-ceramic coated magnesium alloy with strong interfacial bonding strength by “superplasticity diffusion bonding”. Mater. Lett. 2015, 141, 96–99. [Google Scholar] [CrossRef]
- Shen, S.; Cai, S.; Xu, G.; Zhao, H.; Niu, S.; Zhang, R. Influence of heat treatment on bond strength and corrosion resistance of sol-gel derived bioglass-ceramic coatings on magnesium alloy. J. Mech. Behav. Biomed. Mater. 2015, 45, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ASTM. D4541 Standard Test Method for Pull-Off Strength of Coatings Using Portable Adhesion; ASTM Int.: West Conshohocken, PA, USA, 2009. [Google Scholar]
- ASTM International. Available online: https://www.astm.org/Standards/C633 (accessed on 9 September 2019).
- Fletcher, J.F.; Barnes, D.J. Pull-Off Adhesion Testing of Coatings–Improve Your Technique. 2017. Available online: https://www.elcometer.com/images/stories/PDFs/Pull-Off_Adhesion_Testing_of_Coatings_Improve_Your_Technique.pdf (accessed on 5 June 2019).
- Chen, Q.; Cabanas-Polo, S.; Goudouri, O.M.; Boccaccini, A.R. Electrophoretic co-deposition of polyvinyl alcohol (PVA) reinforced alginate-Bioglass® composite coating on stainless steel: Mechanical properties and in-vitro bioactivity assessment. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 40, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Kleinbichler, A.; Pfeifenberger, M.J.; Zechner, J.; Moody, N.R.; Bahr, D.F.; Cordill, M.J. New insights into nanoindentation-based adhesion testing. JOM 2017, 69, 2237–2245. [Google Scholar] [CrossRef]
- Sun, L.; Berndt, C.C.; Gross, K.A.; Kucuk, A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: A review. J. Biomed. Mater. Res. 2001, 58, 570–592. [Google Scholar] [CrossRef]
- Xuereb, M.; Camilleri, J.; Attard, N.J. Systematic review of current dental implant coating materials and novel coating techniques. Int. J. Prosthodont. 2015, 28, 51–59. [Google Scholar] [CrossRef]
- Røynesdal, A.K.; Ambjørnsen, E.; Støvne, S.; Haanaes, H.R. A comparative clinical study of three different endosseous implants in edentulous mandibles. Int. J. Oral Maxillofac. Implant. 1998, 13, 500–505. [Google Scholar]
- Capello, W.N.; D’Antonio, J.A.; Feinberg, J.R.; Manley, M.T. Ten-year results with hydroxyapatite-coated total hip femoral components in patients less than fifty years old. A concise follow-up of a previous report. J. Bone Joint Surg. Am. 2003, 85, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Nelissen, R.G.; Valstar, E.R.; Rozing, P.M. The effect of hydroxyapatite on the micromotion of total knee prostheses. A prospective, randomized, double-blind study. J. Bone Joint Surg. Am. 1998, 80, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Magyar, G.; Toksvig-Larsen, S.; Moroni, A. Hydroxyapatite coating of threaded pins enhances fixation. J. Bone Joint Surg. Br. 1997, 79, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Inadome, T.; Hayashi, K.; Nakashima, Y.; Tsumura, H.; Sugioka, Y. Comparison of bone-implant interface shear strength of hydroxyapatite-coated and alumina-coated metal implants. J. Biomed. Mater. Res. 1995, 29, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Surmeneva, M.A.; Chaikina, M.V.; Zaikovskiy, V.I.; Pichugin, V.F.; Buck, V.; Prymak, O.; Epple, M.; Surmenev, R.A. The structure of an RF-magnetron sputter-deposited silicate-containing hydroxyapatite-based coating investigated by high-resolution techniques. Surf. Coat. Technol. 2013, 218, 39–46. [Google Scholar] [CrossRef]
- Surmeneva, M.A.; Mukhametkaliyev, T.M.; Tyurin, A.I.; Teresov, A.D.; Koval, N.N.; Pirozhkova, T.S.; Shuvarin, I.A.; Shuklinov, A.V.; Zhigachev, A.O.; Oehr, C.; et al. Effect of silicate doping on the structure and mechanical properties of thin nanostructured RF magnetron sputter-deposited hydroxyapatite films. Surf. Coat. Technol. 2015, 275, 176–184. [Google Scholar] [CrossRef]
- ISO Standards. Available online: https://www.iso.org/standard/64617.html (accessed on 9 September 2019).
- Dehghanian, C.; Aboudzadeh, N.; Shokrgozar, M.A. Characterization of silicon- substituted nano hydroxyapatite coating on magnesium alloy for biomaterial application. Mater. Chem. Phys. 2018, 203, 27–33. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Kheradmandfard, M.; Fathi, M.H.; Ahangarian, M.; Zahrani, E.M. In vitro bioactivity evaluation of magnesium-substituted fluorapatite nanopowders. Ceram. Int. 2012, 38, 169–175. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Zhang, L.; Yin, X.; Guo, Y. In simulated body fluid performance of polymorphic apatite coatings synthesized by pulsed electrodeposition. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Filgueiras, M.R.; La Torre, G.; Hench, L.L. Solution effects on the surface reactions of a bioactive glass. J. Biomed. Mater. Res. 1993, 27, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T. Bioactive glass ceramics: Properties and applications. Biomaterials 1991, 12, 155–163. [Google Scholar] [CrossRef]
- Ohtsuki, C.; Kushitani, H.; Kokubo, T.; Kotani, S.; Yamamuro, T. Apatite formation on the surface of Ceravital-type glass-ceramic in the body. J. Biomed. Mater. Res. 1991, 25, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, H.; Gardin, C.; Ferroni, L.; Zavan, B.; Colombo, P.; Bernardo, E. Highly porous Sr/Mg-doped hardystonite bioceramics from preceramic polymers and reactive fillers: Direct foaming and direct ink writing. Adv. Eng. Mater. 2019, 21, 1800900. [Google Scholar] [CrossRef]
- Elsayed, H.; Zocca, A.; Franchin, G.; Bernardo, E.; Colombo, P. Hardystonite bioceramics from preceramic polymers. J. Eur. Ceram. Soc. 2016, 36, 829–835. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.K.; Zheng, X.B.; Xie, Y.T.; Ji, H.; Ding, C.X. Antibacterial properties of vacuum plasma sprayed titanium coatings after chemical treatment. Surf. Coat. Technol. 2009, 204, 685–690. [Google Scholar] [CrossRef]
- Oliveira, W.F.; Silva, P.M.S.; Silva, R.C.S.; Silva, G.M.M.; Machado, G.; Coelho, L.C.B.B.; Correia, M.T.S. Staphylococcus aureus and Staphylococcus epidermidis infections on implants. J. Hosp. Infect. 2018, 98, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.I.; Richards, R.G.; Milz, S.; Schneider, E.; Pearce, S.G. Animal models for implant biomaterial research in bone: A review. Eur. Cell. Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef]
- Wancket, L.M. Animal models for evaluation of bone implants and devices: Comparative bone structure and common model uses. Vet. Pathol. 2015, 52, 842–850. [Google Scholar] [CrossRef]
- Stadlinger, B.; Pourmand, P.; Locher, M.C.; Schulz, M.C. Systematic review of animal models for the study of implant integration, assessing the influence of material, surface and design. J. Clin. Periodontol. 2012, 39, 28–36. [Google Scholar] [CrossRef]






| Metallic Material | E (GPa) | Hv (GPa) | CTE (10−6K−1) | Tm (°C) | ρ (Kg/m3) |
|---|---|---|---|---|---|
| CpTi | 100–105 | 1.520–1.618 | 8.5–9.3 | 1670 | 4510–4520 |
| Ti-6Al-4V | 113–115 | 3.256–3.589 * | 8.7–9.1 * | 1610–1660 | 4430 |
| Ti-6Al-7Nb | 100–110 | 2.648–2.844 * | 8–9.8 | 1530–1590 | 4510–4530 |
| Ti-12Mo-6Zr-2Fe | 63.1–90.1 | 3.207–3.383 * | 8.7–8.89 | 1540–1620 * | 4980–5000 |
| 316L SS | 190–205 | 1.667–2.158 | 15–18 | 1380–1400 | 7870–8070 |
| Mg | 44–45.5 | 0.245–0.490 | 25.5–26.5 | 642–650 | 1730–1750 |
| AZ91 Mg alloy | 44–46 | 0.588–0.598 | 26–26.3 | 482–602 | 1800–1810 |
| ZK61 Mg alloy | 44–46 | 0.824–0.892 * | 25–26 * | 470–530 * | 1803–1840 |
| Bone | 17–22 | 0.196–0.392 * | 10–30 * | 110–130 *§ | 1800–2100 |
| Commercial Name/Material | Composition (in wt.%) | Composition (in mol%) | References |
|---|---|---|---|
| 45S5 Bioglass | 45% SiO2, | 46.1% SiO2, | [92,96,97] |
| 24.5% CaO, | 26.9% CaO, | ||
| 24.5% Na2O, | 24.4% Na2O, | ||
| 6.0% P2O5 | 2.6% P2O5 | ||
| S53P4 | 53% SiO2, | 53.8% SiO2, | [92] |
| 20% CaO, | 21.9% CaO, | ||
| 23% Na2O, | 22.7% Na2O, | ||
| 4% P2O5 | 1.7% P2O5 | ||
| BG_Ca | 46.9% SiO2, | 47.2% SiO2, | [98] |
| 42.3% CaO, | 45.6% CaO, | ||
| 4.7% Na2O, | 4.6% Na2O, | ||
| 6.1% P2O5 | 2.6% P2O5 | ||
| CaK | 46% SiO2, | 47.2% SiO2, | [86] |
| 41% CaO, | 45.6% CaO, | ||
| 7% K2O, | 4.6% K2O, | ||
| 6% P2O5 | 2.6% P2O5 | ||
| 13-93 | 53% SiO2, | 54.6% SiO2, | [92,99] |
| 20% CaO, | 22.1% CaO, | ||
| 6% Na2O, | 6% Na2O, | ||
| 4% P2O5, | 1.7% P2O5, | ||
| 12% K2O, | 7.9% K2O, | ||
| 5% MgO | 7.7% MgO | ||
| Sr-Bioglass | 41.5% SiO2, | 44.5% SiO2, | [92] |
| 18.7% CaO, | 21.5% CaO, | ||
| 26.2% Na2O, | 27.2% Na2O, | ||
| 9.7% P2O5, | 4.4% P2O5, | ||
| 3.9% SrO | 2.4% SrO | ||
| 70S30C | 71.4% SiO2, | 70% SiO2, | [92] |
| 28.6% CaO | 30% CaO | ||
| 58 S | 58% SiO2, | 60% SiO2, | [87] |
| 33% CaO, | 36% CaO, | ||
| 9% P2O5 | 4% P2O5 | ||
| 77 S | 77% SiO2, | 80% SiO2, | [87] |
| 14% CaO, | 16% CaO, | ||
| 9% P2O5 | 4% P2O5 |
| System | Materials | Compositions | CTE (10−6K−1) | E (GPa) | ρ (Kg/m3) | References |
|---|---|---|---|---|---|---|
| Binary oxides | Wollastonite | CaSiO3 | 10–13 | 52 | 2900 | [108,109] |
| Dicalcium silicate | Ca2SiO4 | 8.5 | 10–40 | 3150 | [110,111] | |
| Tricalcium silicate | Ca3SiO5 | _ | 24.9–36.7 | 3210 | [112,113,114] | |
| Dimagnesium silicate | Mg2SiO4 | _ | _ | 3271 | [115] | |
| Magnesium silicate | MgSiO3 | _ | _ | 2600–2800 | [116] | |
| Zinc silicate | Zn2SiO4 | _ | _ | 3300 | [116] | |
| Strontium silicate | SrSiO3 | 10.9 | _ | 3650 | [113,116] | |
| Ternary oxides | Akermanite | Ca2MgSi2O7 | 9.9 | 42–56 | 2961 | [117,118,119] |
| Bredigite | Ca7MgSi4O16 | _ | 43 | 3400 | [120,121] | |
| Diopside | CaMgSi2O6 | 8.4 | 170 | 3200 | [122,123] | |
| Merwinite | Ca3MgSi2O8 | _ | 31–49 | 3150–3330 | [119,124,125] | |
| Hardystonite | Ca2ZnSi2O7 | 11.2 | 37 | 3392 | [118] | |
| Sphene | CaTiSiO5 | 6 | _ | 3539 | [126,127] | |
| Baghdadite | Ca3ZrSi2O9 | _ | 82–120 | 3480 | [128,129] |
| Substrate | Coating Material | Synthesis Method | Deposition Method | Coating Ra (μm) | Average Coating Thickness (μm) | References |
|---|---|---|---|---|---|---|
| CpTi | Sphene | Polymer-derived ceramics route | Airbrushing | 4.1–6.5 | 50–100 | [132] |
| CpTi | Sphene | Polymer-derived ceramics route | Airbrushing | 3.1–8.4 | 133.8 | [133] |
| CpTi | Sphene | Polymer-derived ceramics route | Airbrushing | 0.5–1.4 | 120 | [134] |
| CpTi | Sphene | Polymer-derived ceramics route | Airbrushing | 3.9 | – | [37] |
| Ti-6Al-4V | Sphene | Solid phase reaction | Plasma-spraying | 7.5 | – | [135] |
| Hardystonite | Solid phase reaction | Plasma-spraying | 7.5 | – | ||
| Ti | Sphene | Liquid phase reaction | Micro-arc oxidation | – | ≤21 | [136] |
| Ti-6Al-4V | Sphene | Sol-gel | Dip-coating | – | – | [137] |
| Ti-6Al-4V | Sphene | Sol-gel | Plasma-spraying | 10 | 150 | [127] |
| Ti-6Al-4V | Sphene | Sol-gel | Spin-coating | 0.4 | 0.5–1 | [138] |
| Ti-6Al-4V | Hardystonite | Sol-gel | Plasma-spraying | 12.1 | 170 | [139] |
| Ti-6Al-4V | Hardystonite | Solid phase reaction | Plasma-spraying | 7.7 | 15–18 | [140] |
| Sr-substituted hardystonite | Solid phase reaction | Plasma-spraying | 7.2 | 15–18 | ||
| Ti-6Al-4V | Akermanite | Sol-gel | Plasma-spraying | – | 400 | [141] |
| Ti-6Al-4V | Baghdadite | Solid state reaction | Plasma-spraying | 9.8 | 120 | [142] |
| Ti-6Al-4V | Bredigite | Sol-gel | Plasma-spraying | – | 200 | [143] |
| Ti-6Al-4V | Diopside | Commercially available powder | Plasma-spraying | 8.3 | 200–300 | [123] |
| Ti-6Al-4V | Dicalcium silicate | – | Plasma-spraying | – | 380 | [144] |
| Ti-6Al-4V | Wollastonite | Commercially available powder | Plasma-spraying | – | 350–400 | [145] |
| Ti-6Al-4V | Wollastonite | Liquid phase reaction | Atmosphere plasma spraying (+hydrothermal technology) | – | 120–150 | [53] |
| Ti-6Al-4V | Wollastonite glass-ceramic | Commercially available powder | Thermal spraying | 9 | 100–150 | [146] |
| Wollastonite (36.77 in wt%)-diopside (63.23 in wt%) glass-ceramic | Commercially available powder (wollastonite); solid state reaction (diopside) | Thermal spraying | 11 | 130–200 | ||
| Ti-6Al-4V | Bioactive glass-ceramic with glass phase (SiO2–Al2O3–CaO–P2O5–CaF2) and with fluorapatite (Ca5(PO4)3F) and diopside | Melting and crystallization | Airbrushing | 0.4–1 | 53 | [147] |
| Ti-6Al-4V | Bioactive glass in mol%: 23.41 SiO2, 3.18 CaCO3, 51.45 SrCO3, 8.67 MgO, 4.62 Na2CO3, 4.62 K2CO3, 3.47 ZnO, 5.20 Ca3(PO4)2 | Melting | Plasma-spraying | 11.9 | 50–100 | [148] |
| Ti-6Al-4V | BG Ca | Melting | Plasma-spraying | – | 30–40 | [98] |
| CpTi | Bioactive glass (in mol%: 2.3 K2O, 2.3 Na2O, 45.6 CaO, 2.6 P2O5, 47.3 SiO2) + HA | Melting | High velocity suspension flame spraying | – | 30 | [149] |
| Suspension plasma spraying | – | ≤50 | ||||
| Ti-6Al-4V | CaK | Melting | Pulsed electron deposition | – | 1 | [86] |
| 45S5 Bioglass | Melting | Pulsed electron deposition | – | 1 | ||
| CpTi | 45S5 Bioglass | Melting | High velocity suspension flame spraying | – | 41–83 | [150] |
| Ti | HA + Bioactive glass S53P4 | Commercially available powder | Radio frequent magnetron sputtering | 1.5–2 | 2–3 | [151] |
| Ti | HA + Bioactive glass S53P4 | Commercially available powder | Radio frequent magnetron sputtering | 1.2 | 2.1 | [152] |
| Ti-6Al-4V | Bioactive glass in wt.%: 59.1 SiO2, 19.2 CaO, 5.46 P2O5, 9.4 B2O2, 22.24 Na2O, 1.0 TiO2 | Melting | Vitreous enameling technique | – | 70–100 | [153] |
| Ti grade 4 | x CaO·(1−x)SiO2 bioactive glass (0.0 ≤ x ≤ 0.60) | Sol-gel | Dip-coating | – | – | [1] |
| Ti grade 4 | 70S30CxA bioactive glass (in mol%: 70 SiO2 (S), 30 CaO (C), x Ag2O (A), with 0.08 ≤ x ≤ 0.27) | Sol-gel | Dip-coating | – | – | [154] |
| 316L SS | Hardystonite | Sol-gel | Electrophoretic deposition | – | 14 | [155] |
| 316L SS | Hardystonite | Sol-gel | Electrophoretic deposition | – | – | [156] |
| 316L SS | Wollastonite glass-ceramic | Commercially available powder | Thermal spraying | 10 | 100–150 | [146] |
| Wollastonite (36.77 in wt.%)-diopside (63.23 in wt.%) glass-ceramic | Commercially available powder (wollastonite); solid state reaction (diopside) | Thermal spraying | 13 | 130–200 | ||
| 316L SS | Hybrid organic-inorganic + wollastonite | Sol-gel | Dip-coating | – | 1.1 | [62] |
| 316L SS | Hybrid organic-inorganic + wollastonite | Sol-gel | Dip-coating | – | 1.1 | [157] |
| 316L SS | Hybrid organic-inorganic + 45S5 Bioglass | Sol-gel | Dip-coating | – | 4.2 | [57] |
| Hybrid organic-inorganic + 45S5 Bioglass with Ca partially substituted with 2mol% of Sr | Sol-gel | Dip-coating | – | 4.2 | ||
| Mg alloy (AZ91) | Diopside + bredigite + fluoridated HA | Sol-gel | Anodic spark deposition + electrophoretic deposition | – | – | [66] |
| Mg alloy (AZ91) | Merwinite | Sol-gel | Plasma electrolytic oxidation + electrophoretic deposition | 7 | 250 | [67] |
| Mg alloy (AZ91) | Diopside | Sol-gel | Micro-arc oxidation + electrophoretic deposition | – | – | [65] |
| Mg alloy (ZK60) | Dimagnesium silicate–Magnesium oxide | Liquid phase reaction | Micro-arc oxidation | – | – | [11] |
| Mg alloy (ZK61) | Dimagnesium silicate + Magnesium oxide + Clinoenstatite | Liquid phase reaction | Micro-arc oxidation | – | 10 | [158] |
| Mg alloy (AZ31) | 45S5 glass–ceramic | Sol-gel | Dip-coating | – | 1 | [159] |
| Mg alloy (AZ31) | 45S5 glass–ceramic | Sol-gel | Dip-coating | – | 0.5–1.0 | [160] |
| Mg alloy (AZ31B) | 45S5 glass–ceramic | Sol-gel | Dip-coating | – | – | [161] |
| Mg alloy (AZ31) | 45S5 glass–ceramic | Sol-gel | Dip-coating | – | 1.1 | [162] |
| Mg-Ca (1.4 wt.%) alloy | RKKP * | Liquid phase reaction | Pulsed laser deposition | – | 100 | [69] |
| Substrate | Coating Material | Test Performed | Adhesion Strength (MPa) | References |
|---|---|---|---|---|
| CpTi | Sphene | Scratch test | – | [132] |
| CpTi | Sphene | Scratch test | – | [133] |
| CpTi | Sphene | Scratch test | – | [134] |
| Nanoindentation | – | |||
| Ti-6Al-4V | Sphene | ASTM C-633 | 41.0 ± 3.5 | [135] |
| Hardystonite | ASTM C-633 | 27.0 ± 3.9 | ||
| Ti-6Al-4V | Sphene | ASTM C-633 | 33.2 ± 2.4 | [127] |
| Ti-6Al-4V | Sphene | Scratch test | 17.4 ± 0.9 | [138] |
| Ti-6Al-4V | Hardystonite | ASTM C-633 | 33.4 ± 2.2 | [139] |
| Ti-6Al-4V | Hardystonite | ASTM C-633 | 27 ± 4 | [140] |
| Sr-substituted hardystonite | ASTM C-633 | 35 ± 6 | ||
| Ti-6Al-4V | Akermanite | ASTM C-633 | 38.7–42.2 | [141] |
| Ti-6Al-4V | Baghdadite | ASTM C-633 | 28 ± 4 | [142] |
| Ti-6Al-4V | Bredigite | ASTM C-633 | 41.1–49.8 | [143] |
| Ti-6Al-4V | Diopside | ASTM C-633 | 32.5 ± 2.8 | [123] |
| Ti-6Al-4V | Dicalcium silicate | ASTM C-633 | 38.9 ± 3.5 | [144] |
| Ti-6Al-4V | Wollastonite | ASTM C-633 | 27.4–42.8 | [145] |
| Ti-6Al-4V | Wollastonite glass-ceramic | Microindentation test | – | [146] |
| Wollastonite (36.77 in wt.%)-diopsite (63.23 in wt.%) glass-ceramic | Microindentation test | – | ||
| Ti-6Al-4V | Bioactive glass-ceramic with glass phase (SiO2–Al2O3–CaO–P2O5–CaF2) and with fluorapatite and diopside | Scratch test | – | [147] |
| Ti-6Al-4V | BG_Ca | Scratch test | – | [98] |
| Ti-6Al-4V | CaK | Scratch test | – | [86] |
| 45S5 Bioglass | Scratch test | – | ||
| 316L SS | Wollastonite glass-ceramic | Microindentation test | – | [146] |
| Wollastonite (36.77 in wt.%)-diopside (63.23 in wt.%) glass-ceramic | Microindentation test | – | ||
| Mg alloy (AZ31B) | 45S5 Glass–ceramic | Tensile adhesion test | 14.2–26.8 | [161] |
| Mg alloy (AZ31) | 45S5 Glass–ceramic | Tensile adhesion test | 10.1–27 | [162] |
| Substrate | Coating Material | Control | Soaking Time (days) | Surface Analysis | Ion Release Concentration | Main Results | Reference |
|---|---|---|---|---|---|---|---|
| Ti-6Al-4V | Sphene | – | 21 | SEM, EDS | – | Presence of nanocrystals of apatite on the surface. | [138] |
| Ti-6Al-4V | Hardystonite | – | 28 | SEM, EPMA | – | After 28 days, two layers were present on the coating surface: (a) Top layer: apatite layer, composed of Ca and P with a Ca/P molar ratio ~1.6. (b) Deeper layer: silica-rich layer, perhaps as a consequence of ionic exchange between Ca2+ in the coating and H+ in SBF. | [139] |
| Ti-6Al-4V | Akermanite | – | 2, 6, 14 | SEM, EDS, FTIR | ICP-OES | After two days: some apatite particles on the surface. After six days: thick layer of apatite. After 14 days: apatite layer (3 μm thick), silicon rich layer, original akermanite layer. High weight loss rate over the first six days, then, very low. | [141] |
| Ti-6Al-4V | Baghdadite | – | 14, 28 | SEM, EDS; XRD | – | Apatite formation already obvious after 14 days of immersion. | [142] |
| Ti-6Al-4V | Bredigite | – | 2, 6, 14 | SEM, EDS, FTIR, XRD | ICP-OES | Presence of apatite layer after two days, becoming denser after six days of soaking. After 14 days from outside to inner: apatite layer (thickness ~10 μm), silicon-rich layer and bredigite coating layer. | [143] |
| Ti-6Al-4V | Diopside | – | 5, 15 | SEM, EDS | – | After five days: isolated granular crystals composed of calcium and phosphorous. After 15 days: coating completely covered by apatite layer. | [123] |
| Ti-6Al-4V | Dicalcium silicate | – | 2, 7, 14, 21 | SEM, EDS, XRD | ICP-AES | After two days: a carbonate-containing HA layer was formed on the surface of coating, with the presence of an intermediate silica-rich layer. The thickness of carbonate-containing HA layer increased over time. | [144] |
| Ti-6Al-4V | Wollastonite | Calcium silicate coating (without HT) | 1, 3, 7 | SEM, EDS, XRD, FTIR | _ | HT at 180 °C for 24 h enhanced apatite-mineralization ability of the coatings. | [53] |
| Ti-6Al-4V | Wollastonite glass-ceramic | _ | 7, 14 | SEM, EDS | ICP-AES | Wollastonite glass-ceramic coating exhibited significantly higher dissolution rate than wollatonite-diopsite glass-ceramic coating. | [146] |
| Wollastonite (36.77 in wt%)-diopside (63.23 in wt%) glass-ceramic | |||||||
| Ti-6Al-4V | Bioactive glass-ceramic with glass phase (SiO2–Al2O3–CaO–P2O5–CaF2) and with fluorapatite and diopside | _ | 7, 14, 21 | SEM, EDS | _ | Formation of fluorapatite layer onto the coating surface. Si and Mg elements were significantly increased in the SBF solution with the increase in soaking time. Ca, P and F elements were instead decreased. | [147] |
| Ti-6Al-4V | BG Ca | – | 1, 3, 7, 14 | SEM, EDS, XRD, micro-Raman spectroscopy | – | All the coatings developed a surface layer of hydroxy-carbonated-apatite. The reaction kinetics were influenced by the coatings’ porosity and degree of crystallinity. | [98] |
| CpTi | Bioactive glass (in mol%: 2.3 K2O, 2.3 Na2O, 45.6 CaO, 2.6 P2O5, 47.3 SiO2) + HA | HA | 1, 3, 7, 14 | SEM, XRD, micro-Raman spectroscopy | _ | Porous SPS bioactive glass coatings more rapidly dissolved in SBF, as compared to HVSFS bioactive glass coatings. SPS HA was more stable than HA HVSFS coating | [149] |
| CpTi | 45S5 bioglass | Bulk glass | 1, 3, 7, 14, 28 | SEM, EDS, XRD, micro-Raman spectroscopy | ICP-OES | After one-day presence of HA layer on the sample surface. After 28 days the glass coating was replaced by precipitated HA film. | [150] |
| Ti grade 4 | xCaO·(1−x)SiO2 bioactive glass (0.0 ≤ x ≤ 0.60) | Uncoated | 7, 21 | SEM, EDS | – | After seven days: uncoated samples showed fewer bone-like apatite globular grains in comparison to coated samples. Precipitate increased with the increased in exposure time to SBF. | [1] |
| Ti grade 4 | 70S30CxA bioactive glass (in mol%: 70 SiO2 (S), 30 CaO (C), x Ag2O (A), with 0.08 ≤ x ≤ 0.27) | Uncoated | 21 | SEM, EDS | – | Coated samples showed the surface covered by apatite globular crystals. Coated samples were more bioactive than uncoated ones. | [154] |
| 316L SS | Hardystonite | – | 3, 7, 14 | SEM, EDS, XRD | – | After three days: no changes in coating morphology. After seven and 14 days: presence of cauliflower-shaped apatite on the surface. Iincreasing cracks by the time of immersion. | [155] |
| 316L SS | Wollastonite glass-ceramic | – | 7, 14 | SEM, EDS | ICP-AES | Wollastonite glass-ceramic coating exhibited significantly higher dissolution rate than wollatonite-diopsite glass-ceramic coating. | [146] |
| Wollastonite (36.77% in wt.%)-diopside (63.23% in wt.%) glass-ceramic | |||||||
| 316L SS | Hybrid organic-inorganic + wollastonite | – | 5, 33 | SEM, EDS, XRD | – | An apatite-like layer was observed on the surface, mainly composed of Ca and P. | [62] |
| 316L SS | Hybrid organic-inorganic + wollastonite | – | 5, 33 | SEM, EDX | – | After five days: a Ca-P rich phase was detected in proximity to wollastonite particles. After 33 days: presence of numerous Ca-P rich compounds. | [157] |
| 316L SS | Hybrid organic-inorganic + 45S5 Bioglass | a) Stainless steel; b) double layer of TMS | 30 | SEM, micro-Raman assays | – | Formation of HA on both test surfaces. | [57] |
| Hybrid organic-inorganic + 45S5 Bioglass with Ca partially substituted with 2 mol% of Sr | |||||||
| Mg alloy (AZ91) | Diopside + bredigite + fluoridated HA | a) Coated Mg alloy (ASD/AZ91); b) Mg alloy (AZ91) | 3, 7, 14, 21, 28 | SEM, EDS, FTIR | ICP | Amount of degradation and precipitates on the surface: composite/ASD/AZ91 > ASD/AZ91 > AZ91. | [66] |
| Mg alloy (ZK61) | Dimagnesium silicate + Magnesium oxide + Clinoenstatite | – | 7, 14 | SEM, EPMA, FTIR | – | Quick growing of the apatite layer. | [158] |
| Mg alloy (AZ31) | 45S5 glass–ceramic | Uncoated | 1, 7, 14 | SEM, EDS | – | Enhanced corrosion resistance of coated sample over the first seven days. After 14 days of soaking, reduced corrosion resistance in the coated samples as well due to the cracking of the coating. | [159] |
| Mg alloy (AZ31) | 45S5 glass–ceramic | Uncoated | 1, 3, 5, 7 | SEM, EDS | _ | Samples with the thickest coating, 3A500, showed lower (2.31%) mass loss than A500 (72.71%), 2A500 (72.24%) and uncoated (78.04%) samples, along with a lower pH variation of m-SBF after seven days. | [160] |
| Substrate | Coating Material | Control | Cells | Test Performed | Main Results | References |
|---|---|---|---|---|---|---|
| CpTi | Sphene | Uncoated | hADSCs | - MTT assay - SEM analysis - Immunofluorescence - Alzarin Red S staining - rt-PCR | Sphene-based coating significantly better supported cell attachment and proliferation, than CpTi samples. When cells were seeded in the presence of osteogenic differentiation medium for 21 days, a significantly higher accumulation of calcium deposits on sphene coatings than on uncoated samples was observed. | [37] |
| Ti-6Al-4V | Sphene | Uncoated | Primary human osteoblasts | - SEM analysis - MTS assay - rt-PCR | After seven days of culture, cell proliferation rate on hardystonite coatings was higher when compared with those on sphene coatings and Ti-6Al-4V samples (p < 0.05). Both coatings were able to enhance the expression of bone-related genes. | [135] |
| Hardystonite | ||||||
| Ti-6Al-4V | Sphene | HA-coated (Uncoated) | Human osteoblast-like cells | - SEM analysis - MTS assay - ICP-AES - ALP activity | - After seven days of culture, significantly higher cell proliferation and ALP activity on sphene coatings than on HA-coated and uncoated substrates were observed (p < 0.05). After seven days of culture, no detectable levels of Ti ions and minor amounts of Ca and Si ions released from sphene coatings. | [127] |
| Ti-6Al-4V | Hardystonite | Uncoated | MC3T3-E1 cells (a mouse calvaria-derived osteoblast-like cell line) | - SEM analysis - MTS assay | Hardystonite showed no toxic effect on cells. After 24 h incubation, cells on hardystonite coating were more elongated, spread and confluent than on uncoated samples. | [139] |
| Ti-6Al-4V | Hardystonite | HA-coated | Canine BMMSCs | - Immunofluorescence - rt-PCR - ICP-OES - ALP activity - Calcium deposition assay | After 14 days of culture, the expression levels for BMP-2, ALP and osteocalcin cells cultured on strontium-substituted hardystonite coatings were the highest, followed by hardystonite and then by HA coatings. | [140] |
| Sr-substituted hardystonite | ||||||
| Ti-6Al-4V | Akermanite | HA-coated | Rabbit BMMSCs | - SEM analysis - MTT assay | After one day, cells on HA coating were similar in appearance to those on akermanite coating, but with fewer minor filopodia. After seven days of culture, more cells were detected on the akermanite coating than on the HA one. After one day of culture no significant differences in cell proliferation rate between the two groups; cells on the akermanite coatings showed a higher proliferation rate than that on HA coatings at both three and seven days of culture (p < 0.05 and p < 0.01, respectively). | [141] |
| Ti-6Al-4V | Bredigite | - HA-coated - Blank control | Rabbit BMMSCs | - SEM analysis - MTT assay | Cells cultured on bredigite coating for one day presented an elongated morphology and were firmly attached to the surface. After three days of culture, the bredigite coating presented numerous cells on its surface, characterized by a net-like morphology. After three and seven days of culture, cells on bredigite coating had a higher proliferation rate than that on HA coating and blank control. | [143] |
| Ti-6Al-4V | Wollastonite | – | Rat BMMSCs | - MTT assay - ICP-AES - Immunofluorescence - ALP activity - qRT-PCR | Cells seeded on the HT treated coatings presented higher cell viability and proliferation than untreated coatings at all time points (one, four and seven days) (p < 0.05). Quantitative results of ALP activity cells cultured on HT treated and untreated coatings revealed higher ALP activity on HT treated samples at all time points (four, seven and 10 days) (p < 0.05). HT treatment enhanced the expression of osteogenic genes and angiogenic factors. | [53] |
| CpTi | 45S5 Bioglass | Uncoated | Human osteosarcoma cell line MG63 | - MTT assay - SEM analysis | After 24 h of culture, cells spread over the coated surface. After seven days, it appeared covered by a cell layer. Coated samples supported an increasing cell viability overtime, similarly to uncoated samples. | [150] |
| Ti grade 4 | x CaO·(1 − x)SiO2 bioactive glass (0.0 ≤ x ≤ 0.60) | Uncoated | NIH 3 T3 murine fibroblasts cells | - WST-8 assay | After 24 h of culture, the cells grown on uncoated samples showed lower viability than on all coated samples (p < 0.05). The best results were obtained with 0.3CaO·SiO2 and 0.4CaO·SiO2 coatings, which were homogeneous and crack-free, contrary to SiO2, 0.5CaO·SiO2 and 0.6CaO·SiO2 coatings. | [1] |
| Ti grade 4 | 70S30CxA bioactive glass (in mol%: 70% SiO2 (S), 30% CaO (C), x% Ag2O (A), with 0.08 ≤ x ≤ 0.27 | Uncoated | NIH 3 T3 murine fibroblasts cells | - WST-8 assay | Higher percentage of viable cells on coated samples than on uncoated ones. The coating with the lower content of Ag resulted to be the most biocompatible. | [154] |
| Mg alloy (AZ91) | Diopside + bredigite + fluoridated HA | (a) Uncoated; (b) ASD coated | L-929 fibroblast cell line | - MTT assay - SEM analysis | Increase in cell viability from two to seven days of culture in all samples. At all time points (two, five and seven days) cell viability was as follows: diopside + bredigite + fluoridated HA coated > ASD coated > uncoated. | [66] |
| Mg alloy (AZ91) | Diopside | (a) Uncoated; (b) MAO coated | L-929 fibroblast cell line | - MTT assay | Cell viability of all samples increased with the culture time. At all time points (two, five and seven days) cell viability was as follows: diopside coated > MAO coated > uncoated. Diopside coated samples had significantly higher cell viability than that of uncoated samples at all time intervals (p < 0.05). | [65] |
| Mg alloy (ZK60) | Dimagnesium silicate – Magnesium oxide | Uncoated | Human osteoblast-like cells (MG63) and NIH 3 T3 murine fibroblasts cells | - CellTiter-96 cytotoxicity test - SEM analysis | Dimagnesium silicate-magnesium oxide coatings, with or without gallic acid, favored osteoblast-like cell proliferation. | [11] |
| Substrate | Coating Material | Study Model * | Number of Test Implants | Control Implants § | Sacrifice (wks) | Assessments Method | BIC% | Main Results | References |
|---|---|---|---|---|---|---|---|---|---|
| Ti-6Al-4V | Sphene | Merino sheep (femur) (n = 10) | 20 | (a) Uncoated (n = 20) (b) HA-coated (n = 20) | 6 | - Histological analysis - Histomorphometric analysis - Push-out testing | In cortico-cancellous bone: sphene-coated ~75% - HA coated ~75%, uncoated ~15%. In cortical bone: sphene-coated ~75%, HA coated ~80%, uncoated ~62%. | In cortico-cancellous bone, significantly higher BIC% in sphene- and HA-coated implants, than in uncoated ones. Uncoated implants in corticocancellous site: fibrous tissue and lack of ALP and TRAP staining at the interface. Push-out tests: significantly higher failure load with sphene-coated implants compared to uncoated ones in cortical bone. | [137] |
| Ti-6Al-4V | Hardystonite | Beagle dog (femur) (n = 12) | 12 + 12 | (a) Uncoated (n = 12) (b) HA-coated (n = 12) | 12 | - Sequential fluorescent labeling - Micro-CT analysis - Push-out test - Histomorphometric analysis | Sr-substituted hardystonite 51.20 ± 9.08. hardystonite 36.97 ± 8.72, HA 27.72 ± 5.48, uncoated < 10. | BIC% of Sr-substituted hardystonite-coated implants was higher than those of hardystonite (p < 0.05) and HA (p < 0.01). Push-out test (loading rate of 5 mm/min): Sr-substituted hardystonite-coated implants possessed the highest failure load (388.84 ± 100.51 N). | [140] |
| Sr-substituted hardystonite | |||||||||
| Ti-6Al-4V | Bioactive glass (SrBG) | New Zealand rabbit (femur and tibia) (n = 27) | 54 | HA-coated (n = 54) | 6,12, 24 | - Push-out test - SEM analysis - Histological analysis - Histomorphometric analysis | Quantified using Osteomeasure software (OsteoMetrics) | No significant differences in BIC% between the two groups at any time point. Push-out: significant difference in maximal shear strength at 24 weeks between the two groups (p = 0.028). Maximal shear strength increased over time in bioactive glass-coated samples, but no similar increase in the control group. | [148] |
| Ti | HA + Bioactive glass S53P4 | Beagle dog (mandible) (n = 16) | 16 (HABGHigh) + 16 (HABGLow) | HA-coated (n = 16) | 4, 12 | - Histological analysis - Histomorphometric analysis | At four weeks: HA 41.5 ± 19.7, HABGLow 45.1 ± 19.3, HABGHigh 29.7 ± 12.5. At 12 weeks: overall BIC% ranged from 40.5% to 31.1% with no significant differences between the groups. | After four weeks, in HABGHigh group BIC% was lower than in the other groups (p < 0.05). After 12 weeks, no significant differences in overall BA%, BIC% and first BIC among the groups. | [151] |
| Ti | HA + Bioactive glass S53P4 (HABG) | Saanen goat (iliac crest) (n = 8) | 32 | (a) Uncoated (n = 32) (b) HA-coated (n = 32) | 4 | - Removal torque testing - Histological analysis - Histomorphometric analysis | Monocortical: uncoated 40.7 ± 13.2, HA-coated 44.8 ± 21.7, HABG-coated 54.2 ± 18.4. Bicortical: uncoated 57.5 ± 8.5, HA-coated 65.7 ± 11.3, HABG-coated 66.7 ± 11.5. | HABG-coated implants showed higher (p < 0.05) BIC% in both monocortical and bicortical implant placements in comparison with uncoated implants. | [152] |
| 316L SS | Hybrid organic-inorganic + wollastonite | Hokkaido rat (femur) (n = 4) | Unclear | Uncoated (n unclear) | 8.5 | - Histological analysis - SAXS analysis | ~60 coated | After 60 days, newly formed bone around coated implants and fibrous tissue around uncoated implants. Uniform mean thickness of Ca/P rich crystals in the new bone tissue (~2 nm). | [62] |
| 316L SS | Hybrid organic-inorganic + wollastonite | Hokkaido rat (femur) (n = 4) | Unclear | – | 8.5 | - Surface analysis (SEM, EDS, AFM) - Histological analysis - Nanoindentation | – | After 60 days, newly formed bone around coated implant, characterized by the presence of osteocyte lacunae and laminar structure. | [157] |
| 316L SS | Hybrid organic-inorganic + 45S5 Bioglass | Wistar–Hokkaido rat (femur) (n = 6) | Unclear | Uncoated (n unclear) | 4, 8 | - SEM analysis - Micro-Raman Spectroscopy | – | Thickness of newly formed bone: at eight weeks ~50 μm for all the samples, but at four weeks lower bone thickness around uncoated implants. At four weeks post-op, a better mineralized tissue in samples with Sr-substituted bioactive glass than in those with 45S5 Bioglass coating. | [57] |
| Hybrid organic-inorganic + 45S5 Bioglass with Ca partially substituted with 2 mol% of Sr | |||||||||
| Mg alloy (AZ91) | Merwinite | Rabbit (femur: greatee trochanter) (n = 3) | 1 | (a) Uncoated (n = 1) (b) PEO-coated (n = 1) | 8 | - Blood tests - Radiographs - Histological analysis - Histomorphometric analysis - Measurement of implant weight loss | – | On two-wks post-op radiographs: uncoated samples showed higher gas formation than PEO-coated ones, while no gas on test samples. Two months post-op new bone volume: merwinite (44%) > PEO-coated (31%) > uncoated (27%). Two months post-op: weight loss for uncoated, PEO-coated and merwinite coated implants was 25, 16, and 5 mg/cm2, respectively. | [67] |
| Mg alloy (AZ91) | Diopside | Rabbit (femur: greatee trochanter) (n not specified) | Not specified | (a) Uncoated (n not specified) (b) MAO coated (n not specified) | 8 | - Blood tests - Radiographs - Histological analysis - Histomorphometric analysis - Measurement of implant weight loss | – | No gas formation was clinically observed in any group. On two- weeks post-op radiographs: uncoated samples showed higher gas formation than MAO-coated ones, no gas on test samples. Two months post-op, volume percentage of newly formed bone around implants: diopside coated (65%) > MAO-coated (31%) > uncoated (27%) samples. Two months post-op: the weight loss for uncoated, MAO-coated and diopside coated implants was 25, 16, and 7 mg/cm2, respectively. | [65] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunello, G.; Elsayed, H.; Biasetto, L. Bioactive Glass and Silicate-Based Ceramic Coatings on Metallic Implants: Open Challenge or Outdated Topic? Materials 2019, 12, 2929. https://doi.org/10.3390/ma12182929
Brunello G, Elsayed H, Biasetto L. Bioactive Glass and Silicate-Based Ceramic Coatings on Metallic Implants: Open Challenge or Outdated Topic? Materials. 2019; 12(18):2929. https://doi.org/10.3390/ma12182929
Chicago/Turabian StyleBrunello, Giulia, Hamada Elsayed, and Lisa Biasetto. 2019. "Bioactive Glass and Silicate-Based Ceramic Coatings on Metallic Implants: Open Challenge or Outdated Topic?" Materials 12, no. 18: 2929. https://doi.org/10.3390/ma12182929
APA StyleBrunello, G., Elsayed, H., & Biasetto, L. (2019). Bioactive Glass and Silicate-Based Ceramic Coatings on Metallic Implants: Open Challenge or Outdated Topic? Materials, 12(18), 2929. https://doi.org/10.3390/ma12182929