The Effects of Heat Straightening Temperature on the Microstructure and Properties of 7N01 Aluminum Alloy
Abstract
:1. Introduction
2. Experimental Procedure
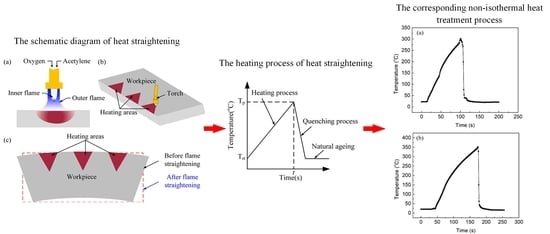
2.1. Heat Treatment Process
2.2. Mechanical Property Experiments
2.3. Corrosion Experiment
2.4. Microstructural Investigations
2.5. Differential Scanning Calorimetry (DSC) Analysis
3. Results
3.1. Mechanical Properties
3.2. Corrosion Behavior
3.3. Microstructure Analysis
3.4. DSC Curve Analysis
4. Discussion
5. Conclusions
- During heat treatment process, most of the initial GP zones dissolve into the matrix, while the grain boundary precipitates have no obvious change. The precipitates of the samples after heat treatment mainly consist of high density GP zones due to the natural aging effect.
- The mechanical properties of 7N01 aluminum alloy decrease after thermal cycling, while there is no obvious difference in the mechanical properties of the specimens after different heat treatment conditions.
- The corrosion resistance of heat treatment samples decrease significantly compared with the base metal, which is mainly related to the change of potential difference between the matrix and grain boundary. The recommended heat straightening temperature is 300 °C.
Author Contributions
Funding
Conflicts of Interest
References
- Li, S.; Dong, H.; Li, P.; Chen, S. Effect of repetitious non-isothermal heat treatment on corrosion behavior of Al–Zn–Mg alloy. Corros. Sci. 2018, 13, 278–289. [Google Scholar] [CrossRef]
- Li, S.; Dong, H.; Shi, L.; Li, P.; Ye, F. Corrosion behavior and mechanical properties of Al–Zn–Mg aluminum alloy weld. Corros. Sci. 2017, 123, 243–255. [Google Scholar] [CrossRef]
- Wang, X.; Mao, S.; Chen, P.; Liu, Y.; Ning, J.; Li, H.; Zang, K.; Zhang, Z.; Han, X. Evolution of microstructure and mechanical properties of a dissimilar aluminium alloy weldment. Mater. Design 2016, 90, 230–237. [Google Scholar] [CrossRef]
- Liu, S.; Li, J.; Mi, G.; Wang, C.; Hu, X. Study on laser-MIG hybrid welding characteristics of A7N01-T6 aluminum alloy. Int. J. Adv. Manuf. Tech. 2016, 87, 1135–1144. [Google Scholar] [CrossRef]
- Lacalle, R.; Álvarez, A.J.; Ferreño, D.; Portilla, J.; Ruiz, E.; Arroyo, B.F. Influence of the flame straightening process on microstructural.; mechanical and fracture properties of S235 JR, S460 ML and S690 QL structural steels. Exp. Mech. 2013, 53, 893–909. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, Z.; Yu, C. Automated flame rectification process planning system in shipbuilding based on artificial intelligence. Int. J. Adv. Manuf. Tech. 2006, 30, 1119–1125. [Google Scholar] [CrossRef]
- Song, F.; Zhang, X.; Liu, S.; Tan, Q.; Li, D. The effect of quench rate and overageing temper on the corrosion behaviour of AA7050. Corros. Sci. 2014, 78, 276–286. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.; Zhou, X.; Hashimoto, T.; Wang, J. The corrosion behaviour of machined AA7150-T651 aluminium alloy. Corros. Sci. 2017, 126, 265–271. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Liu, A. Effect of flame straightening on microstructures and properties of welded joint of aluminium alloy for high-speed train. T. Mater. Heat Treat. 2003, 24, 59–61. [Google Scholar]
- Lu, H.; Shi, L.; Dong, H.; Li, S.; Guo, D.; Tao, C. Influence of flame rectification on mechanical properties of AlZnMg alloy. J. Alloy. Compd. 2016, 689, 278–286. [Google Scholar] [CrossRef]
- Dong, H.; Wang, Y.; Shi, L.; Li, P.; Li, S. Influence of cyclic non-isothermal heat treatment on microstructure, mechanical property and corrosion behavior of Al–Zn–Mg alloy. Mater. Res. Express 2019, 6, 9650. [Google Scholar] [CrossRef]
- Li, S.; Dong, H.; Wang, X.; Liu, Z. Quenching sensitivity of Al–Zn–Mg alloy after non-isothermal heat treatment. Materials 2019, 12, 1595. [Google Scholar] [CrossRef] [PubMed]
- ASTM G110-92. Standard Practice for Evaluating Intergranular Corrosion Resistance of Heat Treatable Aluminum Alloys by Immersion in Sodium Chloride + Hydrogen Peroxide Solution; American Society for Testing Materials (ASTM): West Conshohocken, PA, USA, 2009. [Google Scholar]
- ASTM G34-01. Standard Test Method for Exfoliation Corrosion Susceptibility in 2xxx and 7xxx Series Aluminum Alloys (EXCO Test); American Society for Testing Materials (ASTM): West Conshohocken, PA, USA, 2013. [Google Scholar]
- Liu, S.; Li, C.; Deng, Y.; Zhang, X. Influence of grain structure on quench sensitivity relative to localized corrosion of high strength aluminum alloy. Mater. Chem. Phys. 2015, 167, 320–329. [Google Scholar] [CrossRef]
- Marlaud, T.; Deschamps, A.; Bley, F.; Lefebvre, W.; Baroux, B. Influence of alloy composition and heat treatment on precipitate composition in Al–Zn–Mg-Cu alloys. Acta Mater. 2010, 58, 248–260. [Google Scholar] [CrossRef]
- Nicolas, M.; Deschamps, A. Characterisation and modelling of precipitate evolution in an Al–Zn–Mg alloy during non-isothermal heat treatments. Acta Mater. 2003, 51, 6077–6094. [Google Scholar] [CrossRef]
- Couturier, L.; Deschamps, A.; De Geuser, F.; Fazeli, F.; Poole, W.J. An investigation of the strain dependence of dynamic precipitation in an Al–Zn–Mg-Cu alloy. Scripta Mater. 2017, 136, 120–123. [Google Scholar] [CrossRef]
- Rout, P.K.; Ghosh, M.M.; Ghosh, K.S. Influence of aging treatments on alterations of microstructural features and stress corrosion cracking behavior of an Al–Zn–Mg alloy. J. Mater. Eng. Perform. 2015, 24, 2792–2805. [Google Scholar] [CrossRef]
- Dix, E.H. Acceleration of the rate of corrosion by high constant stresses. Trans. AIME 1940, 137, 11. [Google Scholar]














| Element | Zn | Mg | Mn | Cr | Zr | Fe | Cu | Ti | Si | V | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 4.76 | 1.20 | 0.42 | 0.11 | 0.07 | 0.11 | 0.01 | 0.04 | 0.04 | 0.01 | 93.23 |
| Condition | Immersion Time (h) | ||||
|---|---|---|---|---|---|
| 6 | 12 | 24 | 36 | 48 | |
| Base metal | N | PB | EB | EC | EC |
| 300 °C | N | PC | EB | EC | EC |
| 350 °C | N | PC | EB | EC | EC |
| Condition | Corrosion Potential (mV) | Corrosion Current Density (mA/cm2) |
|---|---|---|
| Base metal | −899 ± 3 | (2.15 ± 0.02) × 10−6 |
| 300 °C | −905 ± 2 | (1.93 ± 0.03) × 10−6 |
| 350 °C | −909 ± 3 | (2.09 ± 0.05) × 10−6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Dong, H.; Shi, L.; Wang, X.; Liu, Z.; Shangguan, L.; Tian, Y. The Effects of Heat Straightening Temperature on the Microstructure and Properties of 7N01 Aluminum Alloy. Materials 2019, 12, 2949. https://doi.org/10.3390/ma12182949
Li S, Dong H, Shi L, Wang X, Liu Z, Shangguan L, Tian Y. The Effects of Heat Straightening Temperature on the Microstructure and Properties of 7N01 Aluminum Alloy. Materials. 2019; 12(18):2949. https://doi.org/10.3390/ma12182949
Chicago/Turabian StyleLi, Shuai, Honggang Dong, Lei Shi, Xingxing Wang, Zhongying Liu, Linjian Shangguan, and Yusong Tian. 2019. "The Effects of Heat Straightening Temperature on the Microstructure and Properties of 7N01 Aluminum Alloy" Materials 12, no. 18: 2949. https://doi.org/10.3390/ma12182949
APA StyleLi, S., Dong, H., Shi, L., Wang, X., Liu, Z., Shangguan, L., & Tian, Y. (2019). The Effects of Heat Straightening Temperature on the Microstructure and Properties of 7N01 Aluminum Alloy. Materials, 12(18), 2949. https://doi.org/10.3390/ma12182949