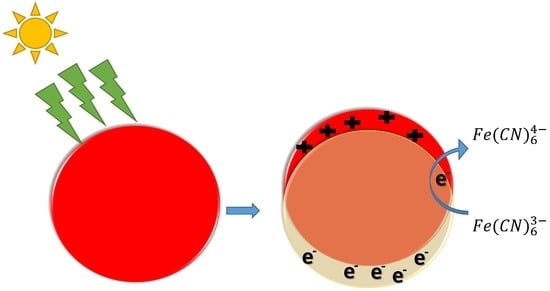
Hexacyano Ferrate (III) Reduction by Electron Transfer Induced by Plasmonic Catalysis on Gold Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Reduction of Hexacyanoferrate in the Presence of Sodium Thiosulfate
3.1.1. Irradiation Using LEDs at λ = 520 nm
- Electron density exchange between the excited gold nanoparticles and nearby reactants;
- Effect of the plasmonic near field enhancement on the electron transfer process between HC-FeIII and ST;
- Temperature increase due to a rapid conversion of the excited surface plasmon resonance into heat that would eventually accelerate the chemical reaction.
3.1.2. Irradiation Using a Xe Lamp Equipped with a 450 nm Optical Cutoff Filter
3.2. Reduction of Hexacyanoferrate in the Absence of Sodium Thiosulfate
3.2.1. Irradiation Using LEDs at λ = 520 nm
3.2.2. Irradiation Using a Xe Lamp Equipped with a 450 nm Optical Cutoff Filter
3.2.3. Effect of the Stabilizing Agent of the Au-NPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krishnendu, S.; Agasti, S.S.; Chaekyu, K.; Xiaoning, L.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar]
- Lang, X.; Chen, X.; Zhao, J. Heterogeneous visible light photocatalysis for selective organic transformations. Chem. Soc. Rev. 2014, 43, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel Gold catalysts for the oxidation of carbon monoxide at a temperature far below 0 °C. Chem. Lett. 1987, 37, 1290–1291. [Google Scholar] [CrossRef]
- Zanella, R.; Giorgio, S.; Shin, C.; Henry, C.; Louis, C. Characterization and reactivity in CO oxidation of gold nanoparticles supported on TiO2 prepared by deposition-precipitation with NaOH and urea. J. Catal. 2004, 222, 357–367. [Google Scholar] [CrossRef]
- Huang, X.; Neretina, S.; El-Sayed, M. Gold nanorods: From synthesis and properties to biological and biomedical applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter. 2017, 29, 203002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.; Ali, S.; Wang, C.; Wang, L.; Meng, X.; Li, B.; Su, D.; Xiao, F. Wet-chemistry strong metal-support interactions in Titania-supported Au catalysts. ACS 2019, 141, 2975–2983. [Google Scholar] [CrossRef]
- Dohyung, K.; Joaquin, R.; Yi, Y.; Asiri, A.M.; Peidong, Y. Synergistic geometric and electronic effects for electrochemical reduction of carbon dioxide using gold-copper bimetallic nanoparticles. Nat. Comm. 2014, 5, 4948. [Google Scholar]
- Abidi, W.; Selvakannan, P.; Guillet, Y.; Lampre, I.; Beaunier, P.; Pansu, B.; Palpant, B.; Remita, H. One-pot radiolytic synthesis of Gold nanorods and their optical properties. J. Phys. Chem. C 2010, 114, 14794–14803. [Google Scholar] [CrossRef]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-Metal Nanostructures for Efficient Conversion of Solar to Chemical Energy. Nat. Mater. 2011, 10, 911–921. [Google Scholar] [CrossRef]
- Kim, K.; Lee, I.; Lee, S.J. Photocatalytic reduction of 4-nitrobenzenthiol on Au mediated via Ag nanoparticles. Chem. Phys. Lett. 2003, 377, 201–204. [Google Scholar] [CrossRef]
- Hong, S.; Li, X. Optimal Size of Gold nanoparticles for Surface-Enhanced Raman Spectroscopy under different conditions. J. Nanomater. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Kowalska, E.; Mahaney, O.O.P.; Abe, R.; Ohtani, B. Visible-light-induced photocatalysis through surface plasmon excitation of gold on titania surfaces. Phys. Chem. Chem. Phys. 2010, 12, 2344–2355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Méndez-Medrano, M.G.; Kowalska, E.; Lehoux, A.; Herissan, A.; Ohtani, B.; Bahena, D.; Briois, V.; Colbeau-Justin, C.; Rodríguez-López, J.L.; Remita, H. Surface Modification of TiO2 with Ag Nanoparticles and CuO Nanoclusters for Application in Photocatalysis. J. Phys. Chem. C 2016, 120, 5143–5154. [Google Scholar] [CrossRef]
- Wang, C.; Astruc, D. Nanogold plasmonic photocatalysis for organic synthesis and clean energy conversion. Chem. Soc. Rev. 2014, 43, 7188–7216. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115–2120. [Google Scholar] [CrossRef]
- Loo, C.; Lin, A.; Hirsch, L.; Lee, M.H.; Barton, J.; Halas, N.; West, J.; Drezek, R. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol. Cancer Res. Treat. 2004, 3, 33–40. [Google Scholar]
- Rong, H.; Wang, Y.-C.; Xiaoyong, W.; Gang, L.; Wei, Z.; Longping, W.; Chen, X.; Kie, Z.; Hou, J.G. Facile synthesis of pentacle gold-copper alloy nanocrystals and their plasmonic and catalytic properties. Nat. Commun. 2014, 5, 4327. [Google Scholar]
- Labouret, T.; Audibert, J.-F.; Pansu, R.B.; Palpant, B. Plasmon-assisted production of reactive oxygen species by single Gold nanorods. Small 2015, 11, 4475–4479. [Google Scholar] [CrossRef]
- Narayanan, R.; El-Sayed, M.A. Effect of catalytic activity on the metallic nanoparticle size distribution: Electron-transfer reaction between Fe(CN)6 and thiosulfate ions catalyzed by PVP-Platinum nanoparticles. J. Phys. Chem. 2003, 107, 12416–12424. [Google Scholar] [CrossRef]
- Yen, C.W.; El-Sayed, M.A. Plasmonic field effect on the hexacyanoferrate (III)-Thiosulfate electron transfer catalytic reaction on gold nanoparticles: Electromagnetic or Thermal? J. Phys. Chem. C 2009, 113, 19585–19590. [Google Scholar] [CrossRef]
- Carregal-Romero, S.; Perez-Juste, J.; Herves, P.; Liz-Marzan, L.M.; Mulvaney, P. Colloidal gold-catalyzed reduction of ferrocyanate (III) by borohydride ions: A model system for redox catalysis. Langmuir 2010, 26, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; El-Sayed, M.A. Shape-dependent catalytic activity of Platinum nanoparticles in colloidal solution. Nano Lett. 2004, 4, 1343–1348. [Google Scholar] [CrossRef]
- Narayanan, R.; El-Sayed, M.A. Changing ctalytic activity during colloidal Platinum nanocatalysis due to shape changes: Electron-transfer reaction. J. Am. Chem. Soc. 2004, 126, 7194–7195. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; El-Sayed, M.A. Effect of nanocatalysis in colloidal solution on the tetrahedral and cubic nanoparticle shape: Electron-transfer reaction catalyzed by Platinum nanoparticles. J. Phys. Chem. B 2004, 4, 5726–5733. [Google Scholar] [CrossRef]
- Sarhid, I.; Abdellah, I.; Martini, C.; Huc, V.; Dragoe, D.; Beaunier, P.; Lampre, I.; Remita, H. Plasmonic catalysis for the Suzuki-Miyaura cross-coupling reaction using Palladium nanoflowers. New J. Chem. 2019, 43, 4349–4355. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–57. [Google Scholar] [CrossRef]
- Frens, G. Particle size and sol stability in metal colloids. Colloid Polym. Sci. 1972, 250, 736–741. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Pluchery, O.; Remita, H.; Schaming, D. Demonstrative experiments about gold nanoparticles and nanofilms: An introduction to nanoscience. Gold Bull. 2013, 46, 319–327. [Google Scholar] [CrossRef]
- Kimling, J.; Maier, M.; Okenve, B.; Kotaidis, V.; Ballot, H.; Plech, A. Turkevich method for gold nanoparticle synthesis revisited. J. Phys. Chem. B 2006, 110, 15700–15707. [Google Scholar] [CrossRef] [PubMed]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics; CRC press: Boca Raton, NY, USA, 2017. [Google Scholar]
- Palpant, B. Photothermal properties of gold nanoparticles. In Gold Nanoparticles for Physics, Biology and Chemistry; Louis, C., Pluchery, O., Eds.; World Scientific: Singapore, 2017; pp. 87–130. [Google Scholar]
- Woehrle, G.H.; Brown, L.O.; Hutchison, J.E. Thiol-functionalized, 1.5-nm gold nanoparticles through ligand exchange reactions: Scope and mechanism of ligand exchange. J. Am. Chem. Soc. 2005, 127, 2172–2183. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.B.; Zhang, M.; Huang, Y.F.; Williams, C.T.; Wu, D.Y.; Ren, B.; Tian, Z.Q. Theoretical study of plasmon-enhanced surface catalytic coupling reactions of aromatic amines and nitro compounds. J. Phys. Chem. Lett. 2014, 5, 1259–1266. [Google Scholar] [CrossRef]
- Yuan, L.; Wenwu, S.; Aditya, G.; Nitin, C. Morphological evolution of gold nanoparticles on silicon wires and their plasmonics. RSC Adv. 2015, 5, 49708–49718. [Google Scholar]
- Cook, R.; Crathorne, E.A.; Monhemius, A.J.; Perry, D.L. XPS study of the adsorption of Gold (I) cyanide by carbons. Hydrometallugy 1989, 22, 171–182. [Google Scholar] [CrossRef]
- Park, J.W.; Shumaker-Parry, J.S. Structural Study of Citrate Layers on Gold Nanoparticles: Role of Intermolecular Intercations in Stabilizing Nanoparticles. J. Am. Chem. Soc. 2014, 163, 1907–1921. [Google Scholar] [CrossRef]
- Huo, Z.; Tsung, C.K.; Huang, W.; Zhang, X.; Yang, P. Sub-Two Nanometer Single Crystal Nanowires. Nano Lett. 2008, 8, 2041–2044. [Google Scholar] [CrossRef] [PubMed]
- Mikhlin, Y.; Likhatski, M.; Karacharov, A.; Zaikowski, V.; Krylov, A. Formation of gold and gold sulfide nanoparticles and mesosclae intermediate structures in the reactions of aqueous HAuCl4 with sulfide and citrate ions. Phys. Chem. Chem. Phys. 2009, 11, 5445–5454. [Google Scholar] [CrossRef]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database, NIST Standard Reference Database. Natl. Ins. Stand. Technol 2000, 20899. [Google Scholar]
- Stefaans, J.G.; Erasmus, E. Electronic effects of metal hexacyanoferrates: An XPS and FTIR study. Mater. Chem. Phys. 2018, 203, 73–81. [Google Scholar]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe2p XPS spectra and bonding in iron compounds. Surf. Interf. Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Y.; Xu, Z. Identifying extraction technology of Gold from solid waste in terms of environmental friendliness. ACS Sustain. Chem. Eng. 2019, 7, 7260–7267. [Google Scholar] [CrossRef]












© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarhid, I.; Lampre, I.; Dragoe, D.; Beaunier, P.; Palpant, B.; Remita, H. Hexacyano Ferrate (III) Reduction by Electron Transfer Induced by Plasmonic Catalysis on Gold Nanoparticles. Materials 2019, 12, 3012. https://doi.org/10.3390/ma12183012
Sarhid I, Lampre I, Dragoe D, Beaunier P, Palpant B, Remita H. Hexacyano Ferrate (III) Reduction by Electron Transfer Induced by Plasmonic Catalysis on Gold Nanoparticles. Materials. 2019; 12(18):3012. https://doi.org/10.3390/ma12183012
Chicago/Turabian StyleSarhid, Iyad, Isabelle Lampre, Diana Dragoe, Patricia Beaunier, Bruno Palpant, and Hynd Remita. 2019. "Hexacyano Ferrate (III) Reduction by Electron Transfer Induced by Plasmonic Catalysis on Gold Nanoparticles" Materials 12, no. 18: 3012. https://doi.org/10.3390/ma12183012
APA StyleSarhid, I., Lampre, I., Dragoe, D., Beaunier, P., Palpant, B., & Remita, H. (2019). Hexacyano Ferrate (III) Reduction by Electron Transfer Induced by Plasmonic Catalysis on Gold Nanoparticles. Materials, 12(18), 3012. https://doi.org/10.3390/ma12183012