Visible-Light Activated Titania and Its Application to Photoelectrocatalytic Hydrogen Peroxide Production
Abstract
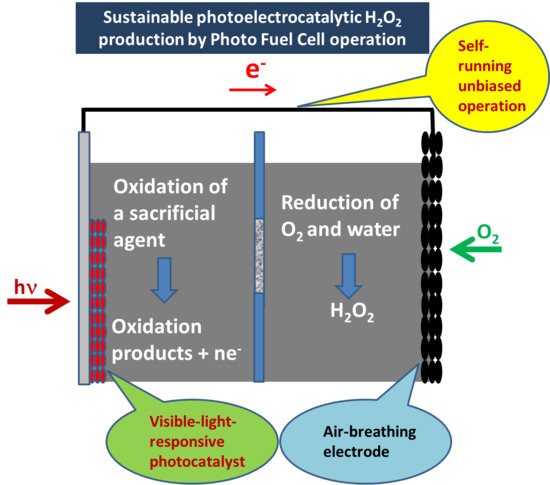
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Construction of the Photoanode
2.2.1. Deposition of the Titania Film
2.2.2. Application of CdS on the Titania Film
2.2.3. Addition of the CdSe Layer and the ZnS Protective Layer
2.3. Construction of the Counter Electrode
2.4. Description of the Reactor
2.5. Measurements
3. Results and Discussion
3.1. Characterization of Electrodes
3.2. Current–Voltage Characteristics of Various Photo Fuel Cells
3.3. Photoelectrocatalytic Hydrogen Peroxide Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, R.; Dutta, S. A review on H2 production through photocatalytic reactions using TiO2/TiO2–assisted catalysts. Fuel 2018, 220, 607–620. [Google Scholar] [CrossRef]
- Lianos, P. Review of recent trends in photoelectrocatalytic conversion of solar energy to electricity and hydrogen. Appl. Catal. B Environ. 2017, 210, 235–254. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for environmental photocatalytic applications: A review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification strategies of TiO2 for potential applications in photocatalysis: A critical review. Green Chem. Lett. Rev. 2018, 11, 86–102. [Google Scholar] [CrossRef] [Green Version]
- Yeoh, M.-E.; Chan, K.-Y. Recent advances in photo-anode for dye-sensitized solar cells: A review. Int. J. Energy Res. 2017, 41, 2446–2467. [Google Scholar] [CrossRef]
- Wang, Z.; Lang, X. Visible light photocatalysis of dye-sensitized TiO2: The selective aerobic oxidation of amines to imines. Appl. Catal. B Environ. 2018, 224, 404–409. [Google Scholar] [CrossRef]
- Sfaelou, S.; Sygellou, L.; Dracopoulos, V.; Travlos, A.; Lianos, P. Effect of the nature of cadmium salts on the effectiveness of CdS SILAR deposition and its consequences on the performance of sensitized solar cells. J. Phys. Chem. C 2014, 118, 22873–22880. [Google Scholar] [CrossRef]
- Antoniadou, M.; Sfaelou, S.; Dracopoulos, V.; Lianos, P. Platinum-free photoelectrochemical water splitting. Catal. Commun. 2014, 43, 72–74. [Google Scholar] [CrossRef]
- Mezzetti, A.; Balandeh, M.; Luo, J.; Bellani, S.; Tacca, A.; Divitini, G.; Cheng, C.; Ducati, C.; Meda, L.; Hongjin Fan, H.; et al. Hyperbranched TiO2-CdS nanoheterostructures for highly efficient photoelectrochemical photoanodes. Nanotechnology 2018, 29, 335404–335415. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Yang, C.-F. Recent advances in the TiO2/CdS nanocomposite used for photocatalytic hydrogen production and quantum-dot-sensitized solar cells. Renew. Sust. Energ. Rev. 2016, 54, 1048–1059. [Google Scholar] [CrossRef]
- Chen, Y.; Chuang, C.-H.; Qin, Z.; Shen, S.; Doane, T.; Burda, C. Electron-transfer dependent photocatalytic hydrogen generation over cross-linked CdSe/TiO2 type-II heterostructure. Nanotechnology 2017, 28, 084002–084012. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.A.; Patilb, D.S.; Junga, H.R.; Parka, J.Y.; Malic, S.S.; Hongc, C.K.; Shinc, J.-C.; Patild, P.S.; Kim, J.-H. Quantum dot sensitized solar cell based on TiO2/CdS/CdSe/ZnS heterostructure. Electrochim. Acta. 2016, 203, 74–83. [Google Scholar] [CrossRef]
- Ai, G.; Li, H.; Liu, S.; Mo, R.; Zhong, J. Solar water splitting by TiO2/CdS/Co-Pi nanowire array photoanode enhanced with Co–Pi as hole transfer relay and CdS as light absorber. Adv. Funct. Mater. 2015, 25, 5706–5713. [Google Scholar] [CrossRef]
- Lin, K.-H.; Chuang, C.-Y.; Lee, Y.-Y.; Li, F.-C.; Chang, Y.-M. Charge transfer in the heterointerfaces of CdS/CdSe cosensitized TiO2 photoelectrode. J. Phys. Chem. C 2012, 116, 1550–1555. [Google Scholar] [CrossRef]
- Liu, J.; Xia, M.; Chen, R.; Zhu, X.; Liao, Q.; Ye, D.; Zhang, B.; Zhang, W.; Yu, Y. A membrane-less visible–light responsive micro photocatalytic fuel cell with the laterally-arranged CdS/ZnS-TiO2 photoanode and air-breathing CuO photocathode for simultaneous wastewater treatment and electricity generation. Sep. Purif. Technol. 2019, 229, 115821. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Chen, Y.-H.; Chen, J.-W.; Cao, F.; Li, L.; Luo, Z.-M.; Leu, I.-C.; Pu, Y.-C. New insights into the electron collection efficiency improvement of CdS–sensitized TiO2 nanorod photoelectrodes by interfacial seed-layer mediation. ACS Appl. Mater. Interfaces 2019, 11, 8126–8137. [Google Scholar] [CrossRef]
- Li, L.; Chen, R.; Zhu, X.; Liao, Q.; Ye, D.; Zhang, B.; He, X.; Jiao, L.; Feng, H.; Zhang, W. A ternary hybrid CdS/SiO2/TiO2 photoanode with enhanced photoelectrochemical activity. Renew. Energy 2018, 127, 524–530. [Google Scholar] [CrossRef]
- Ciriminna, R.; Albanese, L.; Meneguzzo, F.; Pagliaro, M. Hydrogen peroxide: A key chemical for today’s sustainable development. ChemSusChem 2016, 9, 3374–3381. [Google Scholar] [CrossRef]
- Asghar, A.; Raman, A.A.A.; Daud, W.M.A.W. Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef] [Green Version]
- Patton, S.; Romano, M.; Naddeo, V.; Ishida, K.P.; Liu, H. Photolysis of mono- and dichloramines in UV/hydrogen peroxide: Effects on 1,4–dioxane removal and relevance in water reuse. Environ. Sci. Technol. 2018, 52, 11720–11727. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, Y.; Peng, Q.; Wang, Q.; Zhao, G. Catalytic activity of MOF(2Fe/Co)/carbon aerogel for improving H2O2 and OH generation in solar photo-electro-Fenton process. Appl. Catal. B Environ. 2017, 203, 127–137. [Google Scholar] [CrossRef]
- Fujiwara, K.; Akita, A.; Kawano, S.; Fujishima, M.; Tada, H. Hydrogen peroxide-photofuel cell using TiO2 photoanode. Electrochem. Commun. 2017, 84, 71–74. [Google Scholar] [CrossRef]
- Onishi, T.; Fujishima, M.; Tada, H. Solar-driven one-compartment hydrogen peroxide-photofuel cell using bismuth vanadate photoanode. ACS Omega 2018, 3, 12099–12105. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Siroma, Z.; Senoh, H.; Ioroi, T.; Fujiwara, N.; Yasuda, K. A fuel cell with selective electrocatalysts using hydrogen peroxide as both an electron acceptor and A fuel. J. Power Sources 2008, 178, 20–25. [Google Scholar] [CrossRef]
- Mcdonnell–Worth, C.J.; Macfarlane, D.R. Progress towards direct hydrogen peroxide fuel cells (DHPFCS) as an energy storage concept. Aust. J. Chem. 2018, 71, 781–788. [Google Scholar] [CrossRef]
- Han, L.; Guo, S.; Wang, P.; Dong, S. Light-driven, membraneless, hydrogen peroxide based fuel cells. Adv. Energy Mater. 2015, 5, 1400424. [Google Scholar] [CrossRef]
- Papagiannis, I.; Doukasm, E.; Kalarakis, A.; Avgouropoulos, G.; Lianos, P. Photoelectrocatalytic H2 and H2O2 production using visible-light-absorbing photoanodes. Catalysts 2019, 9, 243. [Google Scholar] [CrossRef] [Green Version]
- Shiraishi, Y.; Kanazawa, S.; Kofuji, Y.; Sakamoto, H.; Ichikawa, S.; Tanaka, S.; Hirai, T. Sunlight-driven hydrogen peroxide production from water and molecular oxygen by metal-free photocatalysts. Angew. Chem. Int. Ed. Engl. 2014, 53, 13454–13459. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, Y.; Siahrostami, S.; Zheng, X. Light-driven BiVO4-C fuel cell with simultaneous production of H2O2. Adv. Energy Mater. 2018, 8, 1801158. [Google Scholar] [CrossRef]
- Xiao, K.; Liang, H.; Chen, S.; Yang, B.; Zhang, J.; Li, J. Enhanced photoelectrocatalytic degradation of bisphenol A and simultaneous production of hydrogen peroxide in saline wastewater treatment. Chemosphere 2019, 222, 141–148. [Google Scholar] [CrossRef]
- Ito, S.; Chen, P.; Comte, P.; Nazeeruddin, M.K.; Liska, P.; Pechy, P.; Gratzel, M. Fabrication of screen–printing pastes from TiO2 powders for dye-sensitised solar cells. Prog. Photovolt. Res. Appl. 2007, 15, 603–612. [Google Scholar] [CrossRef]
- Sfaelou, S.; Kontos, A.G.; Falaras, P.; Lianos, P. Micro-Raman, Photoluminescence and Photocurrent studies on the photostability of quantum dot sensitized photoanodes. J. Photochem. Photobiol. A 2014, 275, 127–133. [Google Scholar] [CrossRef]
- Nicolau, Y.F. Solution deposition of thin solid compound films by A successive ionic–layer adsorption and reaction process. Appl. Surf. Sci. 1985, 22/23, 1061–1074. [Google Scholar] [CrossRef]
- Li, Z.; Luo, W.; Zhang, M.; Feng, J.; Zou, Z. Photoelectrochemical cells for solar hydrogen production: Current state of promising photoelectrodes, methods to improve their properties, and outlook. Energy Environ. Sci. 2013, 6, 347. [Google Scholar] [CrossRef]
- Sfaelou, S.; Antoniadou, M.; Dracopoulos, V.; Bourikas, K.; Kondarides, D.I.; Lianos, P. Quantum Dot Sensitized Titania as Visible-light Photocatalyst for Solar Operation of Photofuel Cells. J. Adv. Oxid. Technol. 2014, 17, 59–65. [Google Scholar] [CrossRef]
- Pop, C.L.; Sygellou, L.; Dracopoulos, V.; Andrikopoulos, K.S.; Sfaelou, S.; Lianos, P. One-step electrodeposition of CdSe on nanoparticulate titania filmsand their use as sensitized photoanodes for photoelectrochemicalhydrogen production. Catal. Today 2015, 252, 157–161. [Google Scholar] [CrossRef]
- Kalamaras, E.; Lianos, P. Current Doubling effect revisited: Current multiplication in A Photo Fuel Cell. J. Electroanal. Chem. 2015, 751, 37–42. [Google Scholar] [CrossRef]
- Pop, L.C.; Sfaelou, S.; Lianos, P. Cation adsorption by mesoporous titania photoanodes and its effect on the current-voltage characteristics of photoelectrochemical cells. Electrochim. Acta 2015, 156, 223–227. [Google Scholar] [CrossRef]
- Sayama, K. Production of High-Value-Added Chemicals on Oxide Semiconductor Photoanodes under Visible Light for Solar Chemical-Conversion Processes. ACS Energy Lett. 2018, 3, 1093–1101. [Google Scholar] [CrossRef]
- Shi, X.; Siahrostami, S.; Li, G.L.; Zhang, Y.; Chakthranont, P.; Studt, F.; Jaramillo, T.F.; Zheng, X.; Nørskov, J.K. Understanding activity trends in electrochemical water oxidation to form hydrogen peroxide. Nat. Commun. 2017, 8, 701. [Google Scholar] [CrossRef] [Green Version]








| Time | H2O2 Conc. | H2O2 Mass | Corresp. Molarity | Molar Rate | Equivalent Current | Current at Time | Average Current | Faradaic Efficiency |
|---|---|---|---|---|---|---|---|---|
| (min) | (mg/L) | (mg) | (μmol) | (μmol/min) | (mA) | (mA) | (mA) | % |
| 0 | 0 | 0 | 0 | –– | –– | 10 | – | –– |
| 15 | 250 | 1.25 | 36.8 | 2.45 | 7.87 | 8.5 | 9.3 | 91 |
| 30 | 538 | 2.69 | 79.1 | 2.64 | 8.46 | 7.8 | 8.9 | 95 |
| 45 | 808 | 4.04 | 119 | 2.64 | 8.48 | 7.3 | 8.7 | 98 |
| 60 | 1092 | 5.46 | 161 | 2.68 | 8.59 | 7.2 | 8.6 | 100 |
| 90 | 1672 | 8.36 | 246 | 2.73 | 8.77 | 7.2 | 8.6 | 102 |
| 120 | 2077 | 10.4 | 305 | 2.54 | 8.17 | 7.2 | 8.6 | 95 |
| Time | H2O2 Conc. | H2O2 Mass | Corresp. Molarity | Molar Rate | Equivalent Current | Current at time | Average Current | Faradaic Efficiency |
|---|---|---|---|---|---|---|---|---|
| (min) | (mg/L) | (mg) | (μmol) | (μmol/min) | (mA) | (mA) | (mA) | % |
| 0 | 0 | 0 | 0 | – | – | 6.5 | – | – |
| 30 | 201 | 1.01 | 29.6 | 0.98 | 3.16 | 6.1 | 6.3 | 50 |
| 47 | 329 | 1.65 | 48.4 | 1.03 | 3.30 | 5.1 | 5.8 | 57 |
| 77 | 594 | 2.97 | 87.4 | 1.13 | 3.64 | 4.7 | 5.6 | 65 |
| Time | H2O2 Conc. | H2O2 Mass | Corresp. Molarity | Molar Rate | Equivalent Current | Current at Time | Average Current | Faradaic Efficiency |
|---|---|---|---|---|---|---|---|---|
| (min) | (mg/L) | (mg) | (μmol) | (μmol/min) | (mA) | (mA) | (mA) | % |
| 0 | 0 | 0 | 0 | – | – | 3.8 | – | – |
| 15 | 113 | 0.57 | 16.7 | 1.11 | 3.56 | 3.4 | 3.6 | 99 |
| 30 | 247 | 1.23 | 36.3 | 1.21 | 3.89 | 3.2 | 3.5 | 111 |
| 47 | 415 | 2.07 | 61.0 | 1.30 | 4.17 | 3.2 | 3.5 | 119 |
| 60 | 556 | 2.78 | 81.8 | 1.36 | 4.38 | 3.2 | 3.5 | 125 |
| 75 | 728 | 3.64 | 107 | 1.43 | 4.59 | 3.2 | 3.5 | 131 |
| 95 | 944 | 4.72 | 139 | 1.46 | 4.69 | 3.2 | 3.5 | 134 |
| Time | H2O2 Conc. | H2O2 Mass | Corresp. Molarity | Molar Rate | Equivalent Current | Current at Time | Average Current | Faradaic Efficiency |
|---|---|---|---|---|---|---|---|---|
| (min) | (mg/L) | (mg) | (μmol) | (μmol/min) | (mA) | (mA) | (mA) | % |
| 0 | 0 | 0 | 0 | – | – | 5.1 | – | – |
| 18 | 115 | 0.58 | 16.9 | 0.94 | 3.00 | 5.1 | 5.1 | 60 |
| 40 | 279 | 1.40 | 41.0 | 1.03 | 3.28 | 5.2 | 5.1 | 64 |
| 47 | 352 | 1.76 | 51.8 | 1.10 | 3.52 | 5.2 | 5.2 | 68 |
| 85 | 705 | 3.53 | 104 | 1.22 | 3.90 | 4.9 | 5.0 | 78 |
| Time | H2O2 Conc. | H2O2 Mass | Corresp. Molarity | Molar Rate | Equivalent Current | Current at Time | Average Current | Faradaic Efficiency |
|---|---|---|---|---|---|---|---|---|
| (min) | (mg/L) | (mg) | (μmol) | (μmol/min) | (mA) | (mA) | (mA) | % |
| 0 | 0 | 0 | 0 | – | – | 5.1 | – | – |
| 30 | 20 | 0.10 | 2.9 | 0.10 | 0.31 | 6.2 | 6.3 | 5 |
| 83 | 70 | 0.35 | 10.3 | 0.12 | 0.39 | 5.6 | 5.9 | 7 |
| 125 | 125 | 0.63 | 18.4 | 0.15 | 0.47 | 4.9 | 5.3 | 9 |
| 142 | 152 | 0.76 | 22.3 | 0.16 | 0.50 | 4.6 | 4.7 | 11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos Andrade, T.; Papagiannis, I.; Dracopoulos, V.; César Pereira, M.; Lianos, P. Visible-Light Activated Titania and Its Application to Photoelectrocatalytic Hydrogen Peroxide Production. Materials 2019, 12, 4238. https://doi.org/10.3390/ma12244238
Santos Andrade T, Papagiannis I, Dracopoulos V, César Pereira M, Lianos P. Visible-Light Activated Titania and Its Application to Photoelectrocatalytic Hydrogen Peroxide Production. Materials. 2019; 12(24):4238. https://doi.org/10.3390/ma12244238
Chicago/Turabian StyleSantos Andrade, Tatiana, Ioannis Papagiannis, Vassilios Dracopoulos, Márcio César Pereira, and Panagiotis Lianos. 2019. "Visible-Light Activated Titania and Its Application to Photoelectrocatalytic Hydrogen Peroxide Production" Materials 12, no. 24: 4238. https://doi.org/10.3390/ma12244238
APA StyleSantos Andrade, T., Papagiannis, I., Dracopoulos, V., César Pereira, M., & Lianos, P. (2019). Visible-Light Activated Titania and Its Application to Photoelectrocatalytic Hydrogen Peroxide Production. Materials, 12(24), 4238. https://doi.org/10.3390/ma12244238