1. Introduction
In recent years, with the explosive development of e-commerce platforms and take-out industries, the demand for plastic foam materials has also exploded. Although the government has issued a series of restrictive policies on ‘white plastic garbage’, including ‘plastic limit order’, the 2017 China Express Industry Green Packaging Development Status and Trend Report [
1] pointed out that usage of plastic bags has grown from 3 billion to 22 billion since the ‘plastic limit order’ implementation. Therefore, Plastics Processing Industry Technology Progress 13th Five-Year Development Guidance Opinions [
2] proposed to increase the research and development of energy-saving and environmentally friendly products, accelerate the pace of catching up with foreign advanced technology and production equipment, and further promote the plastics manufacturing industry reform and transformation. A series of actions and policies have indicated that searching for a green material to replace plastic foam products has become a priority.
Foreign researchers use polyvinyl alcohol (PVA), polylactic acid (PLA), and other mainstream adhesion aids as basic additives to explore their composite properties with natural high molecular polymers, such as various plant fibers and starch, and other artificial polymers. The composite performance of materials is much better than that of domestic-related research. Sony Company’s Tsutomu Nojuchi [
3] used waste paper fiber as the main raw material, mixing a certain proportion of micro-polyethylene plastic pellets into modified starch, foamed by water vapor, to prepare foam materials where the compression stress was 0.5 times that of ordinary polypropylene foam. Ganjyal [
4] acetylated ordinary corn starch, adding the obtained cellulose after delignifying by strong alkali, and used ethanol reagent as a foaming agent and talc as a nucleating agent. Meanwhile, material properties were explored by using different concentrations of cellulose. As a result, the compatibility between the plant cellulose and the corn starch is excellent, and the low concentration of cellulose can effectively enhance the physical properties of the foam materials. However, when the concentration reaches 10%, it has a negative impact on the density of the material and the expansion ratio.
Although domestic research started late, many research achievements and breakthroughs in key technologies have been achieved. Zhang of Jiangnan University [
5] used waste corrugated board as the main raw material, modified the fiber with NaOH, and used polyvinyl alcohol (PVA) as the main adhesive to prepare foam materials by low-power microwave foaming technology. The results show that the initial water content of the pulp material is between 90% and 95%, the foam materials have the lowest density and the highest expansion ratio, and the optimum ratio of each ingredient is determined by experiments. Lu et al. [
6] used waste paper fiber and corn starch as the main raw materials and Na
2CO
3-citric acid as the foaming agent, and explored physical properties of the foam materials. Finally, the foam materials with a porosity of 87%, a density of 0.2 g/cm
3, and a Young’s modulus of 700 MPa were obtained. Yang [
7] adopted the melt intercalation method to prepare starch/bean residue composite foaming cushioning material with different contents of MMT. The results indicate that the mechanical strength, water resistance, and compression strength of the composite improve significantly when the mass fraction of montmorillonite (MMT) is 5%.
MMT is a type of clay; it has a unique and natural nanostructure, with a slice scale of nanoscale. Therefore, it is often used in the preparation of nanocomposites. MMT is similar to the effect of nucleating agents, which can increase the number of cells formed in the unit volume during the foaming process. In addition, the layered structure of MMT promotes it to uniformly adsorb on the skeleton structure of the fiber web, and then it becomes the skeleton support structure of the fiber web, so that the cells of the obtained foam materials increase and the pore diameter becomes smaller [
8].
In this work, the formulation and reaction conditions were further optimized based on previous studies [
5]. Furthermore, the MMT was modified and added to the foaming process, where bagasse fiber and corn starch were used as the basic raw materials. Finally, the composite foam materials were prepared by microwave foaming, and the influence of the foaming process condition and the content of various reagents on the properties of the foam materials were investigated. The purpose is to provide a theoretical basis for realizing the industrial production of green degradable materials instead of plastic foam materials as soon as possible.
2. Materials and Methods
2.1. Materials
The bagasse was purchased from local farmers (Yizhou, Guangxi, China), corn starch was bought from Tianjin Kemiou Chemical Reagent Co., Ltd. (Tianjin, China), and sodium tetraborate and calcium carbonate (CaCO3) were purchased from Guangzhou Fuchen Chemical Reagent Co., Ltd. (Guangzhou, China); the above reagents were of analytical purity. MMT K-10 was purchased from Maclean (China) Chemical Reagent Co., Ltd. (Guangzhou, China). MMT is a type of natural mineral of layered silicate with a specific surface area of 240 m2/g and a molecular weight of 30.06900.
Polyvinyl Alcohol (PVA) 350 was purchased from (Wokai) Sinopharm Group (Shanghai, China); ammonium bicarbonate was purchased from Guangzhou Fuchen Chemical Reagent Co., Ltd. (Guangzhou, China); glycerol was purchased from Shanghai Chemical Reagent Co., Ltd. (Shanghai, China); and octadecyldimethylbenzylammonium chloride (ODBA) was purchased from Aladdin Chemical Reagent Co., Ltd. (Shanghai, China); the above reagents were of national standard purity.
2.2. Preparation of Corn Starch-Bagasse Foam Materials
Some bagasse pulp board was immersed in water for about 2 h and then beat for 3000 rpm after spalling; then, some bagasse was placed into a sealed bag at 90 °C, placed in a water bath for 40 min, and kneaded every 10 min. A certain amount of PVA was put into a round bottom beaker and heated in a 90 °C thermostatic heating magnetic stirrer (DF-101S, Gongyi Yuhua Instrument Co., Ltd., Zhengzhou, China) for 40 min until completely dissolved. A small amount of starch was mixed with water with a ratio of 1:3 and stirred constantly in a water bath at 70 °C until it started gelatinizing. The bagasse pulp was mixed with PVA solution and stirred uniformly, then glycerin, calcium carbonate, borax, and starch solution were added. A certain amount of NH
4HCO
3 was added after mixing well. When all were mixed uniformly, the obtained mixture was poured into the mold and heated for a certain time in a microwave oven (M1-L213B, Guangdong Midea Electric Co., Ltd., Guangzhou, China) using 700 W power. Finally, the foam materials were obtained after drying for 12 h when the temperature of the drying oven was set to 40 °C. The specific foaming process is shown in
Figure 1.
In this experiment, NH
4HCO
3 was used as a foaming agent. NH
4HCO
3 was easily decomposed by heating, and the decomposition equation was as follows:
Water was used as a medium for microwave heating. Most of the water in the raw material will be evaporated. As the system was no longer closed during the process of changing from liquid phase to solid phase, NH3 and CO2 will eventually escape from the material. This was the foaming mechanism of this experiment.
2.3. Preparation of OMMT
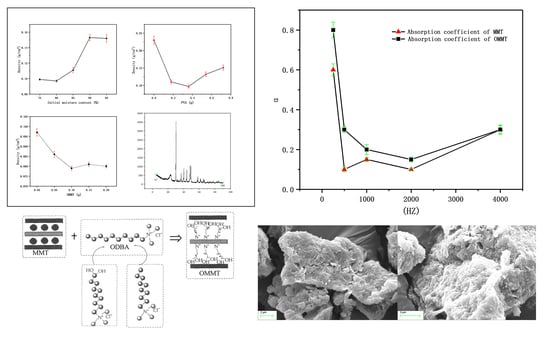
A certain amount of MMT K10 was weighed into a beaker, adding a quantitative amount of 2 mol/L hydrochloric acid solution and adjusting the PH of the solution to 3. ODBA was added into the stirring turbid solution. After heating in an oil bath at 80 °C for 40 min, it was then put in a vacuum oven for one night. Finally, the dry OMMT powder was obtained by grinding fully.
The intercalation method was used in this experiment. It was an important method for preparing polymer/inorganic nanocomposites. Many inorganic compounds, such as silicate clay, phosphates, graphite, metal oxides, disulfides, and phosphorus trisulfide complexes, have a typical layered structure and can be embedded in organic matter. The intercalation method is simple in terms of process and rich and inexpensive in terms of raw materials. The usage of the intercalation polymerization method can improve the mechanical properties of materials reduce costs, and the laminar structure and interface development of MMT can increase the porosity and hygroscopicity of fibers [
9,
10]. In this experiment, an organic quaternary ammonium intercalation agent [
11] was used to modify natural MMT, in order to improve the adsorption of MMT on cellulose and chemical additives. The reaction mechanism is shown in
Figure 2. The methods for MMT modification included cation exchange reactions, silane grafting, and polar polymer adsorption [
12]. In this work, the cationic long carbon chain of the organic quaternary ammonium salt was inserted and covered between the layers of the natural MMT by the ion exchange reaction.
4. Conclusions
In this paper, a method for preparing the low density of the plant fiber foam materials was provided, and the existing formulation and reaction conditions were further optimized.
When the formula ratio was absolute dry bagasse pulp:polyvinyl alcohol (PVA):glycerol:NH4HCO3:corn starch:CaCO3:sodium tetraborate = 9.6:0.36:1.14:0.3:0.3:1:0.1, and the reaction conditions had an initial moisture content of 80% and microwave power of 700 W for running 20 min, the high foam materials with a density of 0.079 g/cm3 were obtained.
MMT was modified by ODBA, and the interlayer spacing of the OMMT was increased from 1.2637 nm to 1.5462 nm. The foam materials obtained by adding the OMMT had a more orderly surface structure and a more uniform internal pore size. When the amount of OMMT was 0.1 g, the dosage was the best and the material density reached the minimum.
In addition, the sound absorption performance of the foam materials was discussed in this work. Under the same curve trend, the foam materials with OMMT had better sound absorption performance than those with MMT, which indicated that the OMMT had an improved effect on the internal pore structure of the foam materials.