RETRACTED: SrFexNi1−xO3−δ Perovskites Coated on Ti Anodes and Their Electrocatalytic Properties for Cleaning Nitrogenous Wastewater
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
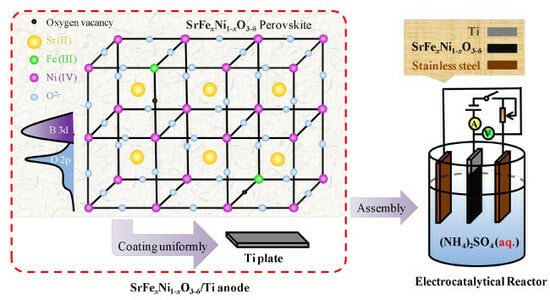
2.2. Fabrication of the SrFexNi1−xO3−δ Perovskites
2.3. Preparation of the SrFexNi1−xO3−δ/Ti Anode
2.4. Assembly of Electrocatalytic Reactor (ECR)
2.5. Measurement of Total Nitrogen (TN) Concentration
2.6. Characterizations and Analysis Methods
2.6.1. Scanning Electron Microscope (SEM) and Energy Dispersive X-ray (EDX) Studies
2.6.2. Fourier Transform Infrared (FT-IR) Studies
2.6.3. Raman Analysis
2.6.4. X-Ray Diffraction (XRD) Studies
2.6.5. Electrochemical Studies
3. Results and Discussion
3.1. Optimum Fabrication Conditions of SrFexNi1−xO3−δ Materials
3.1.1. Effect of Reaction Time for Citrate Sol-Gel on Electrocatalytic Properties of SrFexNi1−xO3−δ
3.1.2. The Effect of Calcination Temperature on the Electrocatalytic Properties of SrFexNi1−xO3−δ
3.1.3. The Effect of the Doping Content of Fe in SrFexNi1−xO3−δ on Electrocatalytic Properties
3.2. The Effect of Electrocatalytic Time on TN Concentration
3.3. Characterization of SrFexNi1−xO3−δ
3.3.1. SEM Studies of SrFexNi1−xO3−δ and SrFexNi1−xO3−δ/Ti Anodes
3.3.2. FT-IR and Raman Analysis of SrFexNi1−xO3−δ
3.3.3. XRD Analysis of SrFexNi1−xO3−δ
3.3.4. EDX Analysis of SrFexNi1−xO3−δ
3.3.5. Electrochemical Impedance Spectroscopy (EIS) and Tafel Curves Analysis of SrFexNi1−xO3−δ
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Camargo, J.A.; Alonso, A. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environ. Int. 2006, 32, 831. [Google Scholar] [CrossRef] [PubMed]
- Bae, B.U.; Jung, Y.H.; Han, W.W.; Shin, H.S. Improved brine recycling during nitrate removal using ion exchange. Water Res. 2002, 36, 3330–3340. [Google Scholar] [CrossRef]
- Xia, G.; Xu, W.; Fang, Q.; Mou, Z.; Pan, Z. Graphene-modulated removal performance of nitrogen and phosphorus pollutants in a sequencing batch chlorella reactor. Materials 2018, 11, 2181. [Google Scholar] [CrossRef] [PubMed]
- Pressley, A.; Bishop, D.F.; Roan, S.G. Ammonia-nitrogen removal by breakpoint chlorination. Environ. Sci. Technol. 1972, 6, 622–628. [Google Scholar] [CrossRef]
- Akira, K.; Guanchen, L.; Michael, V.S.; Yoshihiro, K.; Koichi, K. Modeling the non-equilibrium process of the chemical adsorption of ammonia on gan(0001) reconstructed surfaces based on steepest-entropy-ascent quantum thermodynamics. Materials 2017, 10, 948. [Google Scholar] [CrossRef]
- Huo, S.; Chen, J.; Chen, X.; Wang, F.; Xu, L.; Zhu, F.; Guo, D.; Li, Z. Advanced treatment of the low concentration petrochemical wastewater by Tribonema sp microalgae grown in the open photobioreactors coupled with the traditional Anaerobic/Oxic process. Bioresour. Technol. 2018, 270, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.C.; Bessegato, G.G.; Zanoni, M.V.B. Efficiency comparison of ozonation, photolysis, photocatalysis and photoelectrocatalysis methods in real textile wastewater decolorization. Water Res. 2016, 98, 39–46. [Google Scholar] [CrossRef]
- Özcan, L.; Mutlu, T.; Yurdakal, S. Photoelectrocatalytic degradation of paraquat by Pt loaded TiO2 nanotubes on Ti anodes. Materials 2018, 11, 1715. [Google Scholar] [CrossRef]
- Okanishi, T.; Katayama, Y.; Muroyama, H.; Matsui, T.; Eguchi, K. SnO2-modified Pt electrocatalysts for ammonia-fueled anion exchange membrane fuel cells. Electrochim. Acta 2015, 173, 364–369. [Google Scholar] [CrossRef]
- Reyter, D.; Bélanger, D.; Roué, L. Nitrate removal by a paired electrolysis on copper and Ti/IrO2 coupled electrodes—influence of the anode/cathode surface area ratio. Water Res. 2010, 44, 1918–1926. [Google Scholar] [CrossRef]
- Pinhedo, L.; Pelegrini, R.; Bertazzoli, R.; Motheo, A.J. Photoelectrochemical degradation of humic acid on a (TiO2)0.7(RuO2)0.3 dimensionally stable anode. Appl. Catal. B 2005, 57, 75–81. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, X.; Li, Y.; Chen, L.; Shu, Z.; Chen, H.; Shi, J. High surface area mesoporous LaFexCo1−xO3 oxides: Synthesis and electrocatalytic property for oxygen reduction. Dalton Trans. 2013, 42, 9448–9452. [Google Scholar] [CrossRef]
- Shawahni, A.M.; Abu-Jafar, M.S.; Jaradat, R.T.; Ouahrani, T.; Khenata, R.; Mousa, A.A.; Ilaiwi, K.F. Structural, elastic, electronic and optical properties of SrTMO3 (TM = Rh, Zr) compounds: Insights from FP-LAPW study. Materials 2018, 11, 2057. [Google Scholar] [CrossRef]
- Grabowska, E. Selected perovskite oxides: Characterization, preparation and photocatalytic properties—A review. Appl. Catal. B 2016, 186, 97–126. [Google Scholar] [CrossRef]
- Hwang, J.; Rao, R.R.; Giordano, L.; Katayama, Y.; Yu, Y.; Shao-Horn, Y. Perovskites in catalysis and electrocatalysis. Science 2017, 358, 751–756. [Google Scholar] [CrossRef]
- Shi, Z.; Guo, J.; Chen, Y.; Li, Q.; Pan, Y.; Zhang, H.; Xia, Y.; Huang, W. Lead-free organic-inorganic hybrid perovskites for photovoltaic applications: recent advances and perspectives. Adv. Mater. 2017, 29, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Santos-García, A.J.D.; Solana-Madruga, E.; Ritter, C.; Ávila-Brande, D.; Fabeloc, O.; Sáez-Pucheb, R. Synthesis, structures and magnetic properties of the dimorphic Mn2CrSbO6 oxide. Dalton Trans. 2015, 44, 10665–10672. [Google Scholar] [CrossRef] [PubMed]
- Mori, D.; Oka, H.; Suzuki, Y.; Sonoyama, N.; Yamada, A.; Kanno, R.; Sumiya, Y.; Imanishi, N.; Takeda, Y. Synthesis, structure, and electrochemical properties of epitaxial perovskite La0.8Sr0.2CoO3 film on YSZ substrate. Solid State Ionics 2006, 177, 535–540. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomie distances in halides and chaleogenides. Acta Cryst. 2015, 32, 751–767. [Google Scholar] [CrossRef]
- Ascienzo, D.; Kurt, O.; Greenbaum, S.; Bayer, T.J.M.; Maier, R.A.; Randall, C.A.; Ren, Y. Formation of structural defects and strain in electrodegraded Fe-doped SrTiO3 crystals due to oxygen vacancy migration. J. Am. Ceram. Soc. 2018, 101, 2545–2561. [Google Scholar] [CrossRef]
- Yao, J.; Zhou, M.; Wen, D.; Xue, Q.; Wang, J. Electrochemical conversion of ammonia to nitrogen in non-chlorinated aqueous solution by controlling pH value. J. Electroanal. Chem. 2016, 776, 53–58. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, K.; Hu, C.; Liu, H.; Wang, Y.; Qu, J. Electrochemical oxidation of ammonia accompanied with electricity generation based on reverse electrodialysis. Electrochim. Acta 2018, 269, 128–135. [Google Scholar] [CrossRef]
- Vlyssides, A.G.; Karlis, P.K.; Rori, N.; Zorpas, A.A. Electrochemical treatment in relation to pH of domestic wastewater using Ti/Pt electrodes. J. Hazard. Mater. 2002, B95, 215–226. [Google Scholar] [CrossRef]
- Oskoui, S.A.; Niaei, A.; Tseng, H.H.; Salari, D.; Izadkhah, B.; Hosseini, S.A. Modeling preparation condition and composition-activity relationship of perovskite-type LaxSr1−xFeyCo1−yO3 nano catalyst. ACS Comb. Sci. 2013, 15, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Guo, Y.; Guo, Y.; Lu, G.; Boreave, A.; Retailleau, L.; Baylet, A.; Giroir-Fendler, A. LaMnO3 perovskite oxides prepared by different methods for catalytic oxidation of toluene. Appl. Catal. B 2014, 148–149, 490–498. [Google Scholar] [CrossRef]
- Xu, L.; Xin, Y.; Wang, J. A comparative study on IrO2-Ta2O5 coated titanium electrodes prepared with different methods. Electrochim. Acta 2009, 54, 1820–1825. [Google Scholar] [CrossRef]
- Wang, G.; Gao, J.; Fu, L.; Zhao, N.; Wu, Y.; Takamura, T. Preparation and characteristic of carbon-coated Li4Ti5O12 anode material. J. Power Sources 2007, 174, 1109–1112. [Google Scholar] [CrossRef]
- Vidal-Iglesias, F.J.; Garcia-Araez, N.; Montiel, V.; Feliu, J.M.; Aldaz, A. Selective electrocatalysis of ammonia oxidation on Pt (100) sites in alkaline medium. Electrochem. Commun. 2003, 5, 22–26. [Google Scholar] [CrossRef]
- China Standards Publication. Discharge Standard of Pollutants for Municipal Wastewater Treatment Plant in China; GB 18918-2002; China Standards Press: Beijing, China, 2002. [Google Scholar]
- China Standards Publication. Alkaline Potassium Persulphate Digestion UV Spectrophotometric Method; HJ 636-2012; China Standards Press: Beijing, China, 2012. [Google Scholar]
- Li, B.; Tang, C.; Wang, H.; Zhu, X.; Zhang, Q. An aqueous preoxidation method for monolithic perovskite electrocatalysts with enhanced water oxidation performance. Sci. Adv. 2016, 2, e1600495. [Google Scholar] [CrossRef]
- Hancock, C.A.; Slater, P.R. Synthesis of silicon doped SrMO3 (M = Mn, Co): stabilization of the cubic perovskite and enhancement in conductivity. Dalton Trans. 2011, 40, 5599–5603. [Google Scholar] [CrossRef]
- Tsuruta, A.; Nomura, K.; Mikami, M.; Kinemuchi, Y.; Terasaki, I.; Murayama, N.; Shin, W. Unusually small thermal expansion of ordered perovskite oxide CaCu3Ru4O12 with high conductivity. Materials 2018, 11, 1650. [Google Scholar] [CrossRef] [PubMed]
- Weng, S. Fourier Transform Infrared Spectroscopy; Chemical Industry Press: Beijing, China, 2010; pp. 377–389. [Google Scholar]
- Wu, T.; Xu, Z.; Zhang, Y.; Wang, H.; Cui, C.; Chang, B.; Feng, X.; Liu, W. A pH-responsive biodegradable high-strength hydrogel as potential gastric resident filler. Macromol. Mater. Eng. 2018, 303, 1800290. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Dong, H.L.; Kwon, T.Y.; Park, S.H. Infrared spectra and seebeck coefficient of LnCoO3 with the perovskite structure. J. Solid State Chem. 1994, 112, 376–380. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, D.; Li, J.; Yang, X.; Wu, Y. Effect of Ce on NO direct decomposition in the absence/presence of O2 over La1−xCexSrNiO4 (0 ≤ x ≤ 0.3). J. Mol. Catal. A 2005, 234, 99–105. [Google Scholar] [CrossRef]
- Wang, H.; Li, G.; Li, L. Molten-salt-mediated synthesis and low-temperature electrical conduction of LnCoO3 (Ln = Pr, Nd, Sm, and Gd). J. Alloys Compd. 2014, 612, 227–232. [Google Scholar] [CrossRef]
- Ratheesh, R.; Sreemoolanadhan, H.; Sebastian, M.T. Vibrational analysis of Ba5−xSrxNb4O15 microwave dielectric ceramics resonators. J. Solid State Chem. 1997, 131, 2–8. [Google Scholar] [CrossRef]
- Ye, S.; Wang, C.; Jing, X. Photoluminescence and Raman spectra of double-perovskite Sr2Ca(Mo/W)O6 with A-and B-site substitutions of Eu3+. J. Electrochem. Soc. 2008, 155, J148–J151. [Google Scholar] [CrossRef]
- Fu, M.; Liu, X.; Chen, X. Raman spectra analysis for Ca(B’1/3B”2/3)O3-based complex perovskite ceramics. J. Appl. Phys. 2008, 104, 1182. [Google Scholar] [CrossRef]
- Caracas, R.; Cohen, R.E. Theoretical determination of the Raman spectra of MgSiO3 perovskite and post-perovskite at high pressure. Geophys. Res. Lett. 2006, 33, 229–237. [Google Scholar] [CrossRef]
- Ksepko, E. Perovskite-type Sr(Mn1−xNix)O3 materials and their chemical-looping oxygen transfer properties. Int. J. Hydrogen Energy 2014, 39, 8126–8137. [Google Scholar] [CrossRef]
- Zinkevich, M. Constitution of the Sr-Ni-O system. J. Solid State Chem. 2005, 178, 2818–2824. [Google Scholar] [CrossRef]
- Sun, Z.; Yuan, H.; Liu, Z.; Han, B.; Zhang, X. A highly efficient chemical sensor material for H2S: α-Fe2O3 nanotubes fabricated using carbon nanotube templates. Adv. Mater. 2005, 17, 2993–2997. [Google Scholar] [CrossRef]














| Anodic Materials | Mass Ratio (wt.%) | Function and Characteristic |
|---|---|---|
| SrFexNi1−xO3−δ perovskites | 56 | Active material: stability, attractive electrochemical activity |
| Acetylene black | 7 | Conductor: huge specific surface area |
| Polyvinylidene fluoride (PVDF) | 7 | Binder: antioxidation, hydrophobicity |
| N,N-dimethylacetamide (DMAc) | 30 | Solvent: volatility, low toxicity |
| SrFexNi1−xO3−δ Coated on the Anode | Voltage (V) | Power Density (mW cm−2) | Conductivity of ECR (mS cm−1) | TN Removal Ratio (%) |
|---|---|---|---|---|
| SrNiO3 | 6.97 | 82.0 | 3.38 | 37.21 |
| SrFe0.1Ni0.9O2.95 | 6.68 | 78.6 | 3.52 | 49.75 |
| SrFe0.2Ni0.8 O2.9 | 6.54 | 76.9 | 3.60 | 54.41 |
| SrFe0.3Ni0.7O2.85 | 6.50 | 76.4 | 3.62 | 56.20 |
| SrFe0.4Ni0.6O2.8 | 6.72 | 79.1 | 3.50 | 47.60 |
| SrFe0.5Ni0.5O2.75 | 6.85 | 80.6 | 3.43 | 42.23 |
| Item | Value |
|---|---|
| Anode | SrFexNi1−xO3−δ/Ti, 80.0 mm × 17.0 mm × 1.8 mm |
| Cathode | Stainless steel, 50.0 mm × 20.0 mm × 1.0 mm |
| Electrode gap | 20.0 mm |
| Effective surface area of anode | 5.10 cm2 |
| Perovskite amount coated on the anode | 0.09804 g cm−2 |
| Electrocatalytic current identity | 11.76 mA cm−2 |
| Perovskite material for the anode of ECR | SrFe0.3Ni0.7O2.85 |
| Reaction time for citrate sol-gel | 120 min |
| Calcination temperature for SrFexNi1−xO3−δ | 700 °C |
| Conductivity | 3.62 mS cm−1 |
| Initial TN concentration in wastewater | 150 mg L−1 |
| TN removal ratio | 91.33% |
| Treatment time | 150 min |
| Treatment volume | 300 mL |
| Elements | Weight Percent (%) | Atom Percent (%) |
|---|---|---|
| Sr | 46.02 | 21.53 |
| Ni | 22.44 | 15.67 |
| Fe | 9.85 | 7.23 |
| O | 21.69 | 55.57 |
| Total | 100.00 | 100.00 |
| SrFexNi1−xO3−δ Perovskites | Rct (Ω cm2) | i0 (mA cm−2) |
|---|---|---|
| SrNiO3 | 17.88 | 2.570 × 10−5 |
| SrFe0.1Ni0.9O2.95 | 11.54 | 54.95 × 10−5 |
| SrFe0.2Ni0.8O2.9 | 9.97 | 158.5 × 10−5 |
| SrFe0.3Ni0.7O2.85 | 7.58 | 891.3 × 10−5 |
| SrFe0.4Ni0.6O2.8 | 13.85 | 37.15 × 10−5 |
| SrFe0.5Ni0.5O2.75 | 15.88 | 12.02 × 10−5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Jin, Z.; Chen, L.; Wang, J. RETRACTED: SrFexNi1−xO3−δ Perovskites Coated on Ti Anodes and Their Electrocatalytic Properties for Cleaning Nitrogenous Wastewater. Materials 2019, 12, 511. https://doi.org/10.3390/ma12030511
Zhang Y, Jin Z, Chen L, Wang J. RETRACTED: SrFexNi1−xO3−δ Perovskites Coated on Ti Anodes and Their Electrocatalytic Properties for Cleaning Nitrogenous Wastewater. Materials. 2019; 12(3):511. https://doi.org/10.3390/ma12030511
Chicago/Turabian StyleZhang, Yuqing, Zilu Jin, Lijun Chen, and Jiaqi Wang. 2019. "RETRACTED: SrFexNi1−xO3−δ Perovskites Coated on Ti Anodes and Their Electrocatalytic Properties for Cleaning Nitrogenous Wastewater" Materials 12, no. 3: 511. https://doi.org/10.3390/ma12030511
APA StyleZhang, Y., Jin, Z., Chen, L., & Wang, J. (2019). RETRACTED: SrFexNi1−xO3−δ Perovskites Coated on Ti Anodes and Their Electrocatalytic Properties for Cleaning Nitrogenous Wastewater. Materials, 12(3), 511. https://doi.org/10.3390/ma12030511